Citation Spotlight: ARNO-Arf1 Pathway Regulates RhoA Activity to Control Podosome Formation
- By Cytoskeleton Inc. - Small G-Protein News
- Jul 17, 2017

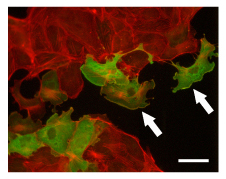
Arf activation by wild-type ARNO GEF in MDCK cells. ARNO proteins localized with a 9e10 anti-myc primary antibody and CY2-conjugated secondary antibody (green). F-actin labeled with rhodamine-phalloidin (red). Cells expressing wild-type ARNO protein have robust lamellipodia (arrows). Scale bar = 50 microns. Image provided by Dr. Lorraine Santy, Penn State University.
Arf activation by wild-type ARNO GEF in MDCK cells. ARNO proteins localized with a 9e10 anti-myc primary antibody and CY2-conjugated secondary antibody (green). F-actin labeled with rhodamine-phalloidin (red). Cells expressing wild-type ARNO protein have robust lamellipodia (arrows). Scale bar = 50 microns. Image provided by Dr. Lorraine Santy, Penn State University.
Recently, Rafiq et al. examined Arf1 control of podosome assembly. Podosomes are actin-rich structures surrounded by adhesion and scaffolding proteins that are involved in cell motility and invasion. Podosomes mediate the adhesion of motile cells to the extracellular matrix and are important for the attachment to, and degradation of, the matrix by motile cells. To better understand podosome formation and maintenance, the contribution of Arf1 and its guanine exchange factors (GEFs), as well as the signaling pathways downstream of Arf1 activation, were evaluated in macrophage-like THP1 cells and fibroblasts. Inhibition of Arf1 or the Arf GEF ARNO with small interfering RNAs (Arf1 and ARNO), pharmacological inhibitors (Brefeldin A [BFA] and SecinH3 for Arf1), or expression of dominant-negative mutants (Arf1 and ARNO) significantly impaired podosome formation and maintenance. Conversely, induction of podosome formation increased levels of active Arf1. Arf1 activity was inversely related to RhoA activity as Arf1 inhibition resulted in increased activation of RhoA and myosin IIA filament assembly. Notably, levels of active Rac1 and Cdc42 were unchanged following manipulation of Arf1 activity. Cytoskeleton’s Cdc42 and Arf1 G-LISA activation assay kits (Cat. # BK127 and BK132, respectively) and cell-permeable Rho inhibitor (Cat. # CT04) were essential reagents in this study, providing the tools necessary to measure the activity of multiple GTPases in a quantitative and sensitive manner.
