Citation Spotlight: Fibronectin Recycling and Fibrillogenesis Regulation by Transforming Growth Factor ß
- By Cytoskeleton Inc. - ECM News
- Jun 14, 2017

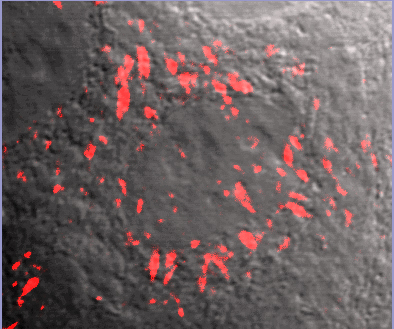
FNR01 image overlay with phase contrast background. Fluorescent fibronectin (Cat. # FNR01) treated MCF10A cells (image kindly provided by A. Varadaraj and M. Karthikenyan, Univ. S.Carolina, Columbia, SC).
Recently, Varadaraj et al. examined transforming growth factor ß (TGF-ß) regulation of the extracellular matrix (ECM) protein fibronectin (FN). Soluble FN dimers polymerize to form insoluble, matrix-associated FN polymers in a process known as fibrillogenesis. The resulting FN fibril network is a scaffold for cell migration, repair, and adhesion mediated by binding to a5ß1 integrin receptors. TGF-ß stimulates ECM remodeling and cell migration through the induction of FN fibrillogenesis, which is necessary for TGF-ß’s effects. Here, the authors found that FN’s role in TGF-ß-mediated ECM remodeling and cell migration can occur via increased FN trafficking, i.e., recycling between the plasma membrane and cytosol. In response to TGF-β, cell surface FN is endocytosed and undergoes Rab11-mediated recycling and subsequent incorporation into fibrils, a process dependent on an interaction between the cytoplasmic domain of the type II TGF-β receptor (TβRII) and a5ß1 integrin. Cytoskeleton’s rhodamine-labeled and biotinylated fibronectin (Cat. # FNR01 and FNR03, respectively) were essential reagents in this interesting study, providing the tools necessary to discover that TGF-ß induces FN trafficking/recycling, a novel process that offers a rapid pathway by which FN can regulate cell migration, wound repair, and fibrosis.
Human SOS1 Protein (Exchange Domain 564-1049) (Cat. # CS-SOS1)
Rhodamine Fibronectin (Cat. # FNR01)
Biotinylated Fibronectin (Cat. # FNR03)
