Citation Spotlight: Nanometer Scale Temperature Measurements of Myosin ATPase Activity
- By Cytoskeleton Inc. - Actin News
- Jul 27, 2017

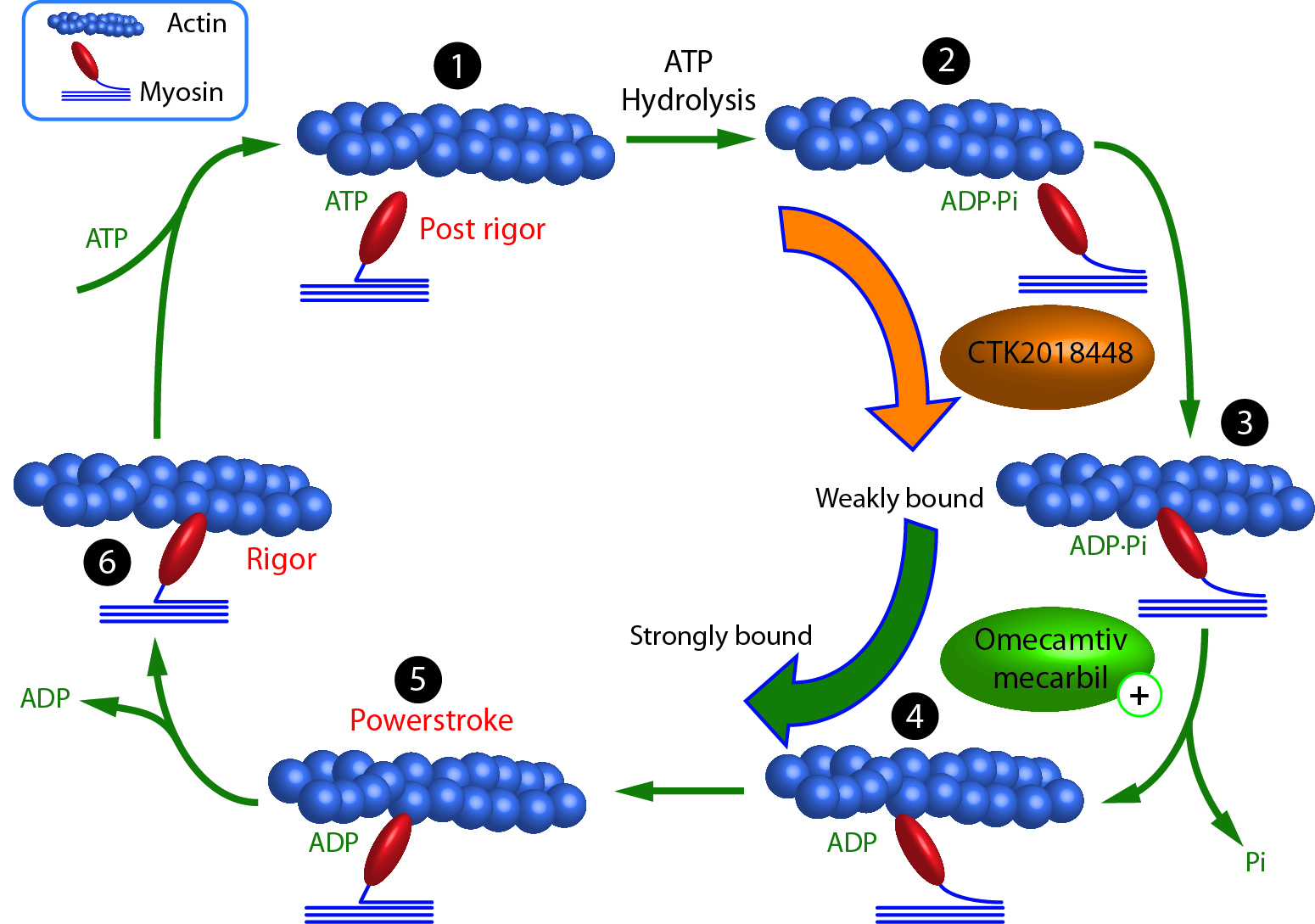
Laha et al. recently developed a new thermometric approach for measuring temperature changes of individual proteins on a nanometer (nm) scale. Spatial and temporal resolution of 80 nm and 1 mK, respectively, was achieved by attaching 2 nm cadmium telluride quantum dots (CdTe QDs) directly to bovine cardiac and rabbit skeletal muscle myosins. The goal was to demonstrate that temperature changes of individual motors performing work (i.e., ATP hydrolysis) can be quantified by measuring the corresponding fluorescence intensity shifts of temperature-sensitive CdTe QDs. Heat released by myosin-mediated ATP hydrolysis was quantified as a means of calculating efficiency since heat loss is inversely related to work performed. Using this nanothermometry, rabbit skeletal myosin was more efficient than bovine cardiac myosin at ATP hydrolysis. Nanometer scale sensitivity significantly improved muscle efficiency measurements toward the goal of single cell thermometry to support efforts to provide early diagnosis and treatment of muscle and metabolic diseases on a nanoscale level. Cytoskeleton’s CytoPhos Phosphate Assay, rabbit skeletal muscle myosin, and bovine cardiac muscle myosin (Cat. # BK054, MY02 and MY03 respectively) were essential reagents in this study, providing the tools necessary to measure muscle efficiency as a function of motor's temperature change during work.
Link to citation:
Laha S.S. et al. 2017. Nanothermometry measure of muscle efficiency. Nano Lett. 17, 1262-1268.