* Limited stock available. If stock is not available, Cytoskeleton will produce a new batch upon request. Minimum order will apply. Inquire for more information.
Product Uses
- IC50 & EC50 determinations for anti-tubulin ligands.
- Characterization of tubulin binding proteins.
Materials
Tubulin protein has been purified from Caki-1 tumor tissue by an adaptation of the method of Davis et al. (2), using anion exchange chromatography followed by a cycle of polymerization/depolymerization. TM001 is supplied in 250 μg aliquots in a lyophilized format in 10 mM Na-PIPES buffer pH 6.9, 0.25 mMMgCl2, 0.1 mM GTP, 5% sucrose and 1% Ficoll-400K. Tubulin consists of a heterodimer of one alpha and one beta isotype, eachtubulin isotype is 55 kDa in size, SDS-PAGE analysis shows tubulin running as a 55 kDa species (see Figure 2). Typically, the molar equivalent of tubulin is defined as the heterodimer which has a molecular weight of 110 kDa.

Figure 2. Purity Analysis of Tubulin Protein. A 100 μg sample of TM001 protein was separated by electrophoresis in a 4-20% SDS-PAGE system,and stained with Coomassie Blue. Protein quantitation was performed using the Precision Red Protein Assay Reagent (Cat. # ADV02). Molecular weight markers are from Invitrogen (Mark 12). Note: Due to overloading of the gel, the tubulin band appears to run lower than the 55 kDa markerband. Purity of TM001 was determined to be >90%.
Storage
On arrival, it is recommended that TM001 is stored in a desiccated chamber at <10% at 4°C, where it is stable for 6 months. After reconstitution in 50 μl of ice cold freshly made G-PEM plus 10% glycerol and 0.05% Triton X-100, the protein is stable for 1h on ice. If aliquots are needed for future experiments then resuspend to 4mg/ml with 62.5 μl of the same buffer and snap freeze experimental sized aliquots in liquid nitrogen and store at -70°C. Aliquots of T234S MUST be snap frozen in liquid nitrogen prior to storage at -70°C, failure to do this results in significant loss of activity. Likewise defrosting tubulin solution should be rapid byplacing the tube in room temperature water for 1min then transferto ice.
Biological Activity Assay
The biological activity of TM001 is assessed by a tubulin polymerization assay. The ability of tubulin to polymerize into microtubules can be followed by observing an increase in fluorescence using 10μM Dapi in the G-PEM buffer plus 10% glycerol with 2mg/ml tubulin (see Figure 3). Under these experimental conditions the fluorescence will increase by 15-20% over 15 minutes at 37°C (see Figure 3). The assay volume is 10 μl and a low volume 384well plate is used (Corning # 3676).
Reagents
1. Tubulin protein (Cat. # TM001)
2. GTP stock (100 mM) (Cat. # BST06)
3. Tubulin Buffer (G-PEM); 80 mM PIPES pH 6.9, 2 mM MgCl2, 0.5 mM EGTA and 1 mM GTP (made fresh or from frozenstock)
Equipment
1. Temperature regulated fluorescence plate reader at 37°C on kinetic mode at Excitation 360 nm, and Emission 410 or420nm.
2. Low volume 384-well plate (Corning Cat # 3676)
Method
1. Place one TM001 vial on ice.
2. Resuspend in 125 μl of G-PEM plus 10% glycerol and 0.05%Triton X-100.
3. Prepare compounds for screening at 5x concentration in PEMplus 5% DMSO.
4. Pipette 2 μl compound into each well of a pre-warmed 37°C plate.
5. Pipette 10 μl of tubulin solution into each well and start theplate reader protocol. Measure tubulin polymerization by takingreadings every 30 seconds for 30 min.
6. Figure 3 shows the results of polymerizing TM001 under the conditions described above.
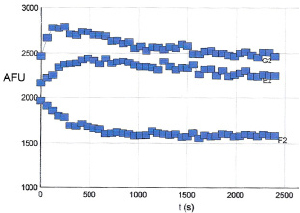
Figure 3. Tubulin Polymerization Assay Polymerizations were carried out as indicated in the Method section. Polymerization was started by incubation at 37°C and followed by fluorescence read at 360nmEx / 410nmEm. Under these conditions the control reaction reached a maximal fluorescence after 1000 seconds (middle curve). Top curve contains 10μM taxol whereas the lower curve contains 10 μM vinblastine.
Important Technical Notes when Working with Tubulin protein
1. Any buffer containing GTP should be kept on ice and used within 1-2h after addition of GTP as GTP will hydrolyze overtime. Unused GTP supplemented buffer should be discarded.
2. Tubulin is a labile protein and should be used immediately after thawed or snap frozen into appropriate aliquots (see Storage section). Freeze/thaw cycles should be avoided. Keep tubulinon ice prior to beginning the polymerization reaction.
3. Temperature is an extremely important parameter for tubulin polymerization. Temperatures cooler than 37°C will significantly decrease the rate and final OD340nm reading of a polymerization reaction. If tubulin is aliquoted into a cool plate (or room temperature plate) there will be a much longer nucleation phase (Figure 3).
4. Polymerization conditions can be altered to optimize a given assay requirement. For example, to examine polymerization enhancers such as taxol, it is recommended to reduce the tubulin concentration to 1 3 mg/ml and polymerize in buffer without glycerol. These conditions will result in a very slow andshallow polymerization curve for the “no compound” control. In this case, efficient polymerization is achieved by addition of an enhancer such as taxol (5 - 10 μM final concentration).
References
1. Amos, LA. & Klug A. 1974. J. Cell Sci. 14: 523-530.
2. Davis A. et al. 2010. Mets. Cell Biol., 97, Ch 18, p331-351.
For product Datasheets and MSDSs please click on the PDF links below.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
Coming soon! If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com


