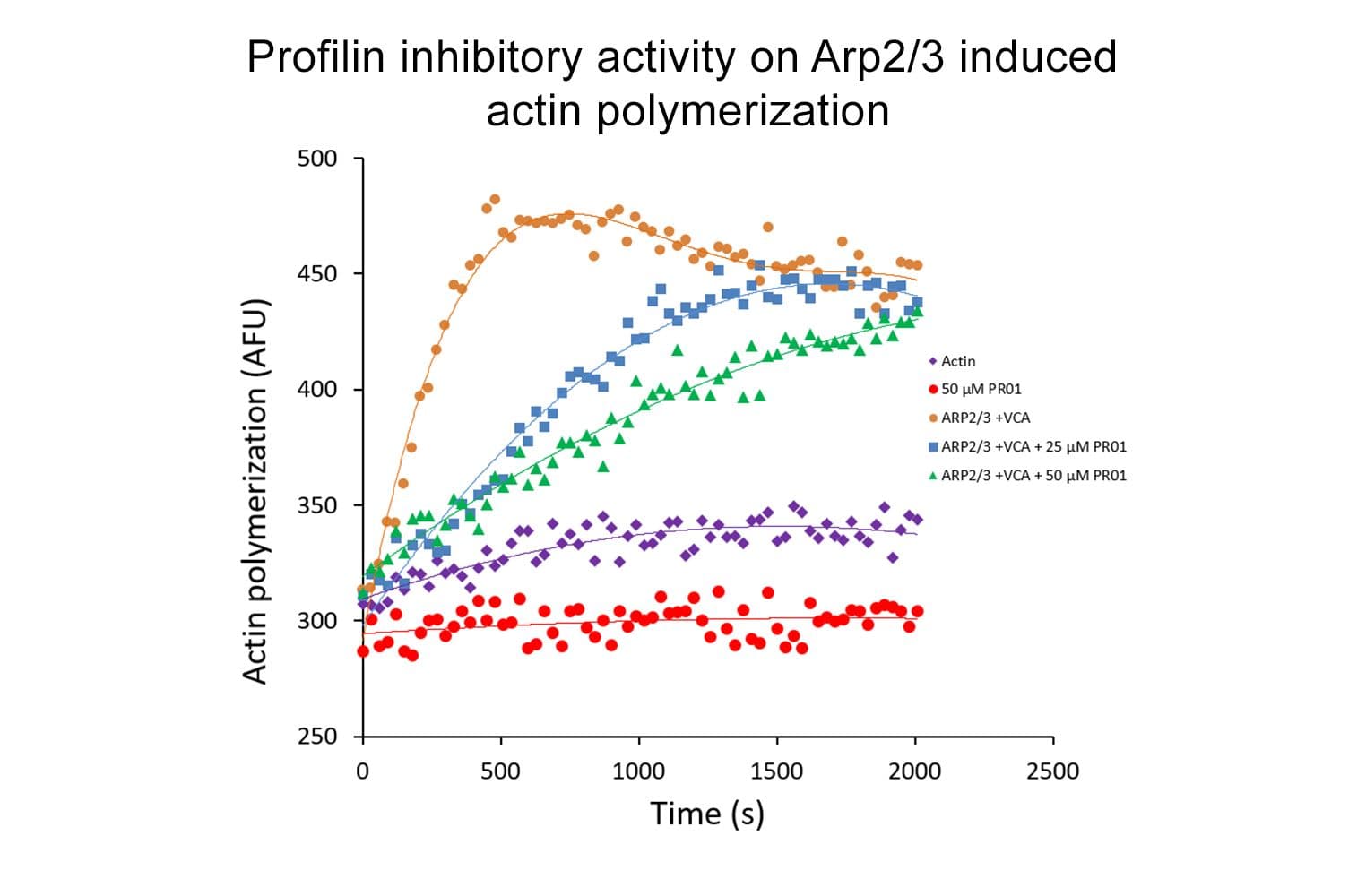
Profilin is a small globular actin-binding protein capable of binding actin monomers with micromolar affinity at a stoichiometry of 1:1. Depending on conditions and molar ratios of actin to profilin, profilin can act to enhance or inhibit actin polymerization. It plays a crucial role in cell motility, signaling, and cytoskeletal organization across eukaryotic organisms.
The human profilin1 protein has been produced in a bacterial expression system.
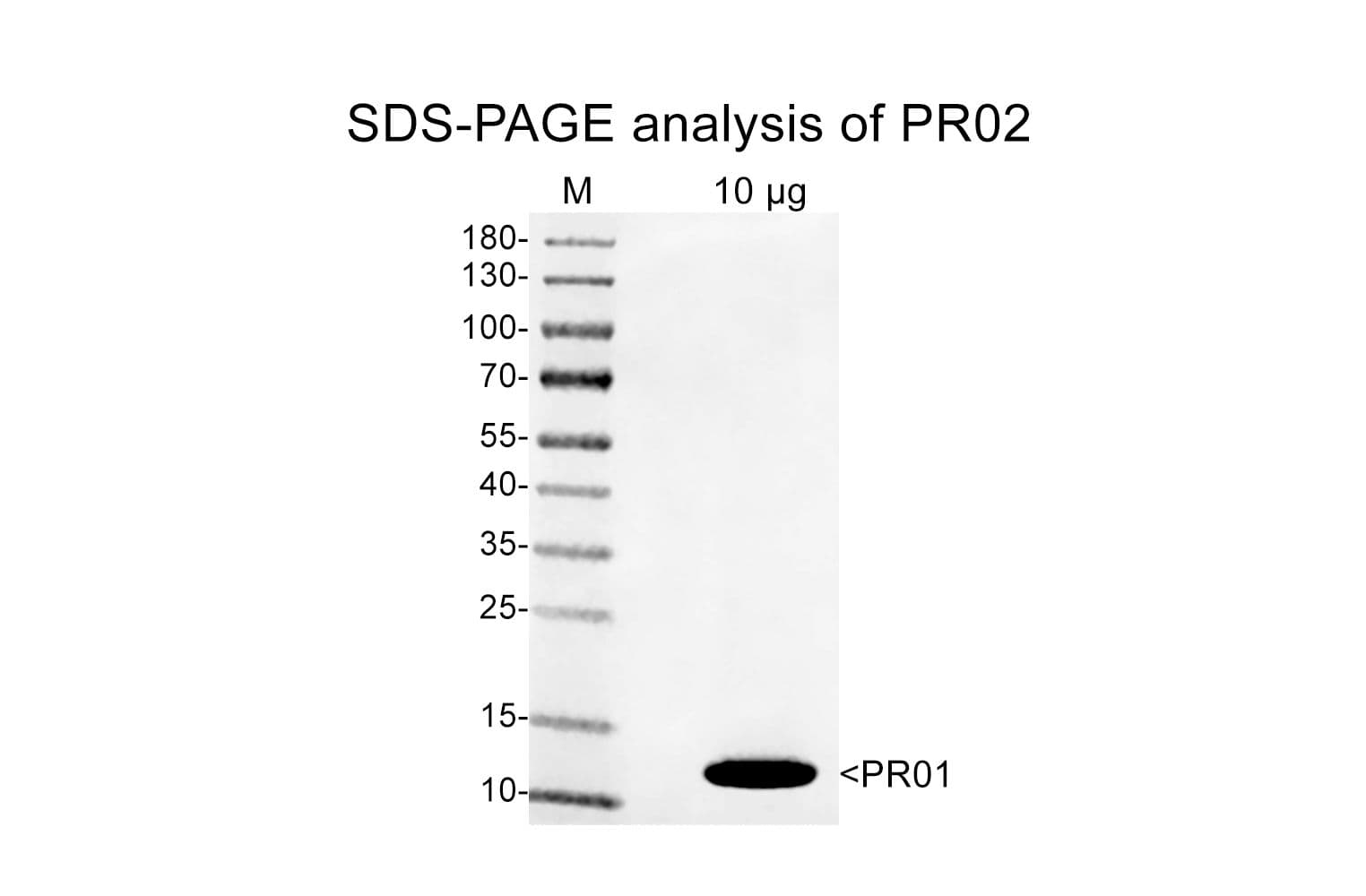
Protein purity is determined by scanning densitometry of Coomassie Blue-stained protein on a 4-20% polyacrylamide gel. Purity is ≥95%
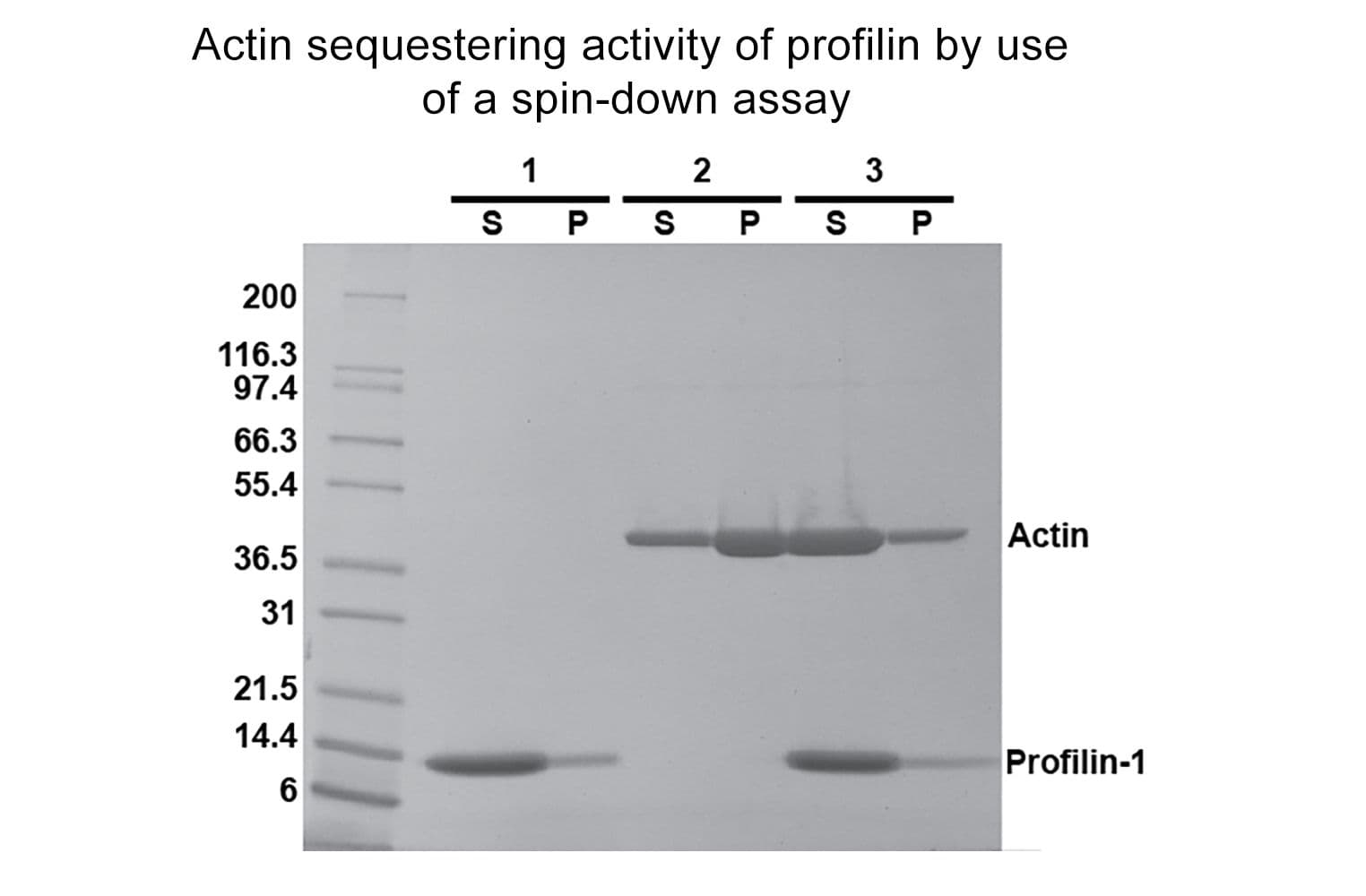
The biological activity of profilin is determined by its ability to inhibit actin polymerization. G-actin is incubated with and without profilin before the addition of actin polymerization buffer. F-actin is separated from G-actin by centrifugation, and the proportion of actin in the supernatant (G-actin) versus the pellet (F-actin) is compared to a control reaction without profilin. Under the assay conditions (see datasheet for details), profilin (15 µg) inhibits actin (10 µg) polymerization by ≥60%. Quality control ensures these binding/severing patterns are consistently observed by SDS-PAGE analysis.
Cat. #PR02