RhoA is a small GTPase that regulates actin cytoskeleton dynamics, primarily promoting the formation of stress fibers and focal adhesions to support cell contractility and adhesion. Through downstream effectors such as ROCK and mDia, RhoA controls actin filament bundling, myosin II activation, and the stabilization of actin structures critical for cell shape and migration.
Wild-type human RhoA protein is produced in a bacterial expression system.
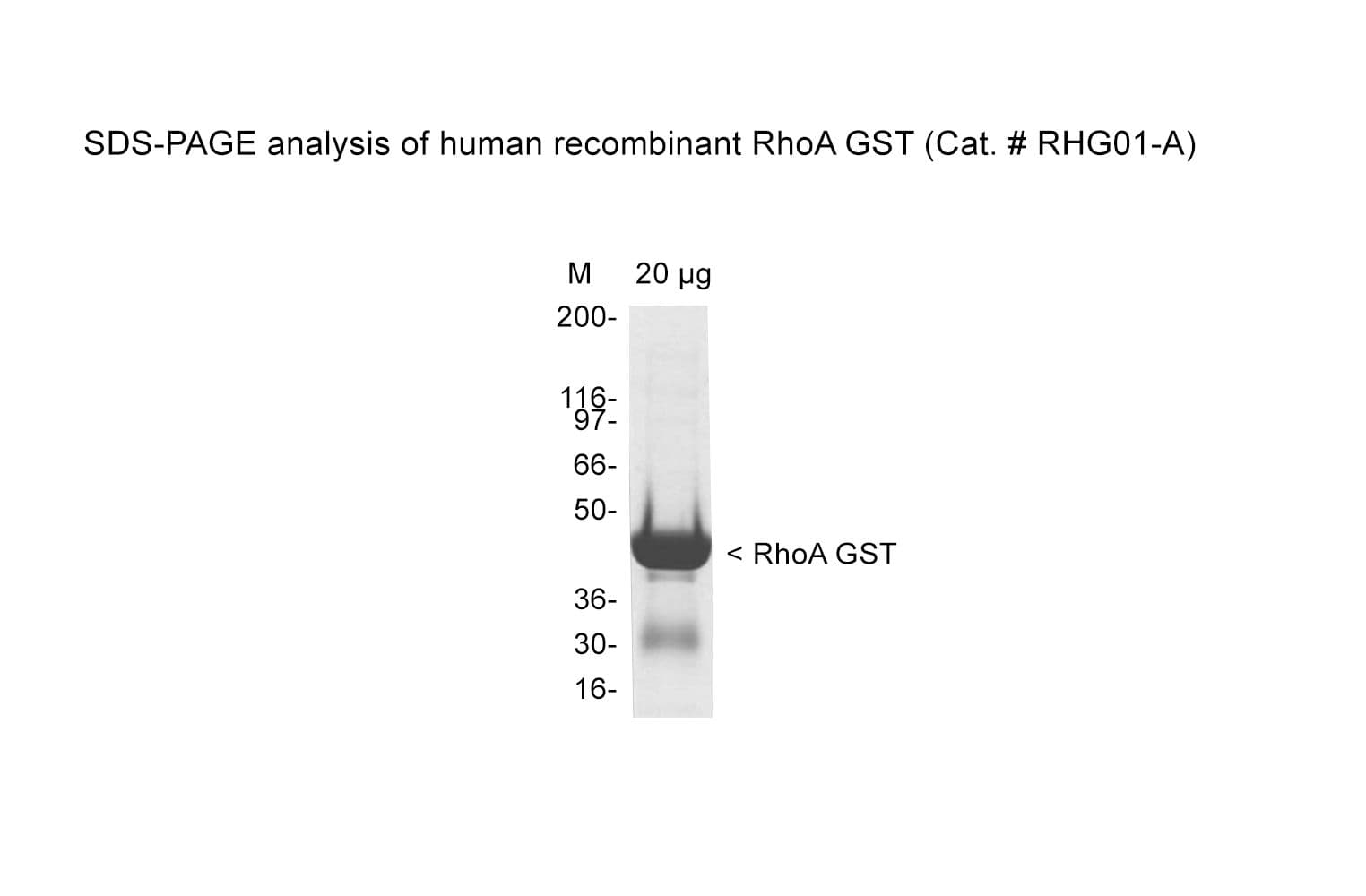
Protein purity is assessed by scanning densitometry of Coomassie Blue-stained protein on a 4-20% polyacrylamide gel. Purity was determined to be ≥90% pure.
The biological activity of RHG01 is evaluated by measuring its GTPase activity—its ability to hydrolyze GTP. Strict quality control ensures that GST-RhoA exhibits ≥2 fold increase in GTP hydrolysis rate in the presence of the Rho GAP, hDbs GE01.
Cat. #RHG01-C