Newsletter 4/05/22: Oncogenic RAS Signaling And Tumor Immune Evasion: Mechanisms And Therapeutic Opportunity
Newsletter 2/06/22: The Cytoskeleton's Role in Autism Spectrum Disorder
Newsletter 11/02/21: The Rac1 and Cdc42 Pathway Axis in Future Metastatic Cancer Therapy
Newsletter 6/08/21: KRAS - No Longer Undruggable
Newsletter 3/16/21: Small G-Proteins and Microvesicle-Mediated Cancer Biogenesis
Newsletter 1/12/21: Cytoskeleton's Activation Assays and Their Utility in Several Studies
Newsletter 1/10/20: Rho GTPases Control Cell Migration
Newsletter 9/30/19: RhoB GTPase Tumor Supporter or Suppressor
Newsletter 8/06/19: Rac1B, Cancer, and Rac1
Newsletter 7/19/19: Rho-Family GTPases, Neuronal Plasticity, and Depression
Newsletter 8/06/18: Rab GTPases and Neurodegeneration
Newsletter 6/05/18: Why Does K-Ras Display Oncogenic Specificity?
Newsletter 4/03/18: Rho Family GEFs and Dendritic Spine Structural Plasticity
Newsletter 1/16/18: The GEF Trio and Its Role in Autism Spectrum Disorders
Newsletter 5/16/17: Arf6 GEFs and Cancer Cell Invasion and Metastasis
Newsletter 11/16/16: Small Molecule Inhibitors of GEF-Mediated GTPase Signaling
Newsletter 8/03/16: Tyrosine Phosphorylation Regulates Rho Family GTPase Activity
Newsletter 4/18/16: Rac1 in Diabetes: The Good and Bad
Newsletter 9/25/15: Dr. Alan Hall - GTPase Legacy
Newsletter 5/01/15: Ras Cancer Therapeutics: 5 Promising Targets
Newsletter 3/26/15: YAP1 Grabs the SPotlight in Oncogenic Ras Addiction
Newsletter 1/26/15: Phosphorylation of RhoA as a Regulator of Signal Transduction
Newsletter 1/19/15: GTPase Activation Assays: Detecting Different Isoforms
Newsletter 7/01/14: New Inhibitors to Control Ras Signaling Via Sos/K-Ras Binding
Newsletter 7/01/14: Rho GTPases and Reactive Oxygen Species: Crosstalk and Feedback
Newsletter 9/18/13: Ras and Rho Post-Translational Modification by Prenylation
Newsletter 1/04/13: Dendritic Spines: Role of Arf6 in Development
Newsletter 10/01/12: Ubiquitination and the Regulation of Rho Family Pathways
Newsletter 9/04/12: The Role of Rac1 GTPase in Neurodegeneration
Newsletter 7/31/12: Epithelial-Mesenchymal Transition (EMT) and the Involvement of Rho Family Small G-Proteins
Newsletter 3/31/12: Trafficking: Arfs and the Cdc42/Rac Connection
Newsletter 9/1/11: New Small G-Protein Tools
Newsletter 10/12/11: Smalll G-Protein and Activation Assay News
For more specific information about Small G-Protein tools please view our product pages and datasheets.
Also included in this newsletter:
- Ras, Rac, and Rho Tools
- Related Publications
Also included in this newsletter:
GLISA and Pulldown Activation Assays
Related Publications
Also included in this newsletter:
GLISA and Pulldown Activation Assays
Related Publications
Although the RAS family of proteins was discovered nearly four decades ago there has not been a viable drug therapy developed to effectively blocks mutant RAS dysfunction. Targeting RAS directly is difficult because of the absence of known allosteric regulatory sites, as well as the fact that it has picomolar affinity to GTP/GDP (3). Alternative drugs have been developed to target Farnesyl transferase and downstream MEK targets, but these have failed for various reasons (reviewed in (1, 2)). Recently, a drug that targets mutant RAS G12C specifically has shown promising clinical results and is now the first FDA approved RAS-targeting drug for the treatment of NSCLC, read on for a summary of this drugs journey from discovery to approval, and roadblocks that still lie ahead.
- Custom Proteins, Custom Services, Ras Activation Assays
- Related Publications
Under control of guanine nucleotide exchange factors (GEFs), small G-proteins cycle between the inactive GDP-bound state and the active GTP-bound state; thus, acting as molecular switches in a diverse multitude of cellular processes1. Small G-proteins have been implicated as central modulators of biological processes including cell growth, cell differentiation, and cell movement2,3. It is now widely recognized that aberrant activity of small G-proteins belonging to the Ras superfamily, which were initially characterized as tumor oncoproteins, contribute heavily to the role of human diseases such as cancer4. Their importance is so integral that scientists continue to link small G-proteins to a wide range of cellular processes involved in tumor progression and metastasis.
Microvesicles (MVs), a type of extracellular vesicle (EV) shed from the outward blebbling of cellular plasma membranes with a diameter of 100-1000 nm, play a role in oncogenesis5. Although the underlying mechanisms of MV generation and release are not completely understood, the impact of microvesicular cargo is well known.
Also included in this newsletter:
- Activation Assays, Kits, Beads, Pulldown Kits.
- Related Publications
Small G-proteins act as molecular switches in a diverse multitude of cellular processes. Distinct from their larger heterotrimeric G protein cousins, small G proteins are monomeric, but share the highly conserved GTPase domain. GTPases cycle between the inactive GDP-bound state and the GTP-bound active state; the latter of which induces a conformational change¹. Several small G proteins belonging to the Ras superfamily were characterized as tumor oncoproteins and have received impressive scrutiny by the scientific community due to their cell signaling roles in overrepresented human diseases such as inflammation, neurodegenerations, inflammatory syndrome, and cancer²-⁴. This newsletter aims to highlight recent Ras superfamily and Rho subfamily discoveries facilitated by Cytoskeleton’s activation assays, which enable investigators to specifically detect the GTP-bound active state of small G proteins.
Also included in this newsletter:
- Actin Biochem Kits, Pull-Down Kits, GLISA Kits
- Related Publications
Directed cell migration depends upon integrin-containing focal adhesions connecting the cell’s actin cytoskeleton with the extracellular matrix (ECM) and transmitting mechanical force. Focal adhesion formation and subsequent migration require dynamic re-organization of actin-based contractile fibers and protrusions at a cell’s trailing and leading edge, respectively, in response to extracellular guidance cues. Migration is essential for healthy cell (and organism) development, growth, maturation, and physiological responses to diseases, injuries, and/or immune system challenges. Various pathophysiological conditions (e.g., cancer, fibrosis, infections, chronic inflammation) usurp and/or compromise the dynamic physiological processes underlying migration1-5.
Rho-family GTPases (e.g., RhoA, Rac1, Cdc42, and RhoJ) act as molecular switches, cycling between a GTP-bound “on” state and a GDP-bound “off” state. Activation is mediated by guanine nucleotide exchange factors (GEFs) and intrinsic GTPase activity is amplified/activated by GTP-activating proteins (GAPs). Rho-family GTPases regulate the dynamic assembly and disassembly of actin filaments which are necessary for the formation, extension, withdrawal, and disassembly of the cellular protrusions (i.e., filopodia and lamellipodia; regulated by Rac and Cdc42, respectively) at the front of the cell and of contractile actomyosin fibers (regulated by RhoA) at the rear of the cell1-8 (Fig. 1). Interestingly, recent research clearly demonstrates that RhoA is also active at the leading edge1,9-12. Of particular interest is how these GTPases regulate cell migration and force generation through the dynamic re-organization of the actin cytoskeleton1-3,13,14 (Fig. 1). This newsletter discusses the roles of Rho-family GTPases in establishing and regulating cell-ECM adhesions and cellular directionality during cell migration.
Also included in this newsletter:
- Tubulin and Actin Live Cell Reagents, G-LISA Activation Assay Kits, Tubulin Kits, Actin Biochem Kits
- Related Publications
RhoB is a Rho-family GTPase that regulates essential physiological processes such as cell division, morphology, motility, adhesion, and intracellular transport, primarily through dynamic remodeling of the actin cytoskeleton, and whose expression and/or activity is pathologically dysfunctional in human diseases such as cancer and neurodegenerative diseases1-3. Due to its unique C-terminal region and distinct post-translational modifications there, RhoB is localized not only to the plasma membrane (like other Rho GTPases), but also to endosomes, multivesicular bodies, and even the nucleus1,3 (Fig. 1). Like other Rho-family GTPases, RhoB functions as a binary switch in signaling cascades, cycling between a GDP-bound, inactive state and a GTP-bound, active state. The GTP/GDP cycling is controlled by guanine nucleotide exchange factors (GEFs; activation by exchanging GDP for GTP) and GTPase-activating proteins (GAPs; inactivation by GTP hydrolysis)1-3.
RhoB expression and/or activity is regulated by a variety of physiological stimuli. Normally expressed at low levels under steady state conditions, RhoB expression and/or activity is rapidly up-regulated by hypoxia, growth factors, inflammatory cytokines, and stress stimuli including UV radiation1,3-8 (Fig. 1). Upon activation, RhoB regulates cellular responses to UV-induced DNA damage, apoptosis, cell cycle progression, and cell migration (and invasion in the case of cancer cells)1,3. RhoB’s distinctive subcellular localization to membrane vesicles enables RhoB-mediated regulation of intracellular transport. Endosome-localized RhoB regulates the trafficking (and thereby function) of receptor tyrosine kinase and cytokine receptor-mediated signaling cascades (e.g., EGFR, CXCR2, TNFR) and the activities of kinases such as Src and Akt9-13 (Fig. 1). As a result, RhoB is able to regulate a wide range of essential signaling cascades involved in cellular development, proliferation, survival, and apoptosis – all pathways important in human physiology and disease1,3.
Also included in this newsletter:
- Tubulin and Actin Live Cell Reagents, G-LISA Activation Assay Kits, Tubulin Kits, Actin Biochem Kits
- Related Publications
The Rac1B GTPase is an alternative splice variant of Rac1, and both GTPases are members of the Rho sub-family of the Ras super-family of GTPases. Insertion of 19 additional amino acids (a.k.a. exon 3b) in Rac1B confers faster GEF-independent GDP/GTP nucleotide exchange due to reduced affinity for GDP and reduced intrinsic GTP hydrolysis compared to Rac11-3(Fig. 1). Biochemically, Rac1B behaves similarly to a constitutively-active Rac1 GTPase, but through a mechanism distinct from oncogenic Rho sub-family GTPase mutants4. Rac1B participates in many phases of oncogenesis, including regulation of cell cycle progression and increased resistance to apoptosis. Additionally, the GTPase has multiple roles in tumor progression, including malignant transformation, epithelial-mesenchymal transition (EMT), metastasis, and invasion3. This newsletter briefly discusses some of the more recent findings regarding the role of Rac1B in tumorigenesis and its relationship with Rac1.
Rac1B is widely regarded as a pro-tumorigenic GTPase as studies find that Rac1B promotes cellular transformation in NIH3T3 mouse fibroblasts and may enhance tumor progression in cancers that over-express Rac1B (e.g., colorectal cancer, human lung adenocarcinomas, thyroid carcinomas)3,5. Rac1B’s role in tumorigenesis involves activation of pathways that result in chronic inflammation, transcription of pro-proliferative genes, inhibition of signaling pathways which inhibit growth and stimulate cell cycle arrest, and modulation of signaling pathways that reduce cell adhesion and promote cell migration3(Fig 2). Rac1B is also an important player in matrix metalloproteinase-3 (MMP3)-induced EMT in breast, lung, and pancreas carcinomas3,6-8. Conversely, Rac1B can also exert anti-tumor effects in certain cancers (e.g., pancreatic ductal adenocarcinomas [PDAC]). In PDAC-derived cells, Rac1B inhibited EMT induced by TGF-β1, as determined by measuring changes in cell morphology, gene expression of EMT markers, signaling cascades downstream of TGF-β1 activation, cell migration, and growth inhibition following RNAi-induced exon 3b-targeted knockdown of Rac1B3,9,10. In addition, Rac1B offers potential as a therapeutic as different GTPase inhibitor compounds are selective for it over Rac111. In addition, Rac1B may serve as a prognostic marker for some cancers (e.g., breast, colorectal, hepatocellular carcinoma, non-small cell lung cancer, pancreatic cancer, chronic pancreatitis, and thyroid cancer)12-14.
Also included in this newsletter:
- Tubulin and Actin Live Cell Reagents, G-LISA Activation Assay Kits, Tubulin Kits, Actin Biochem Kits
- Related Publications
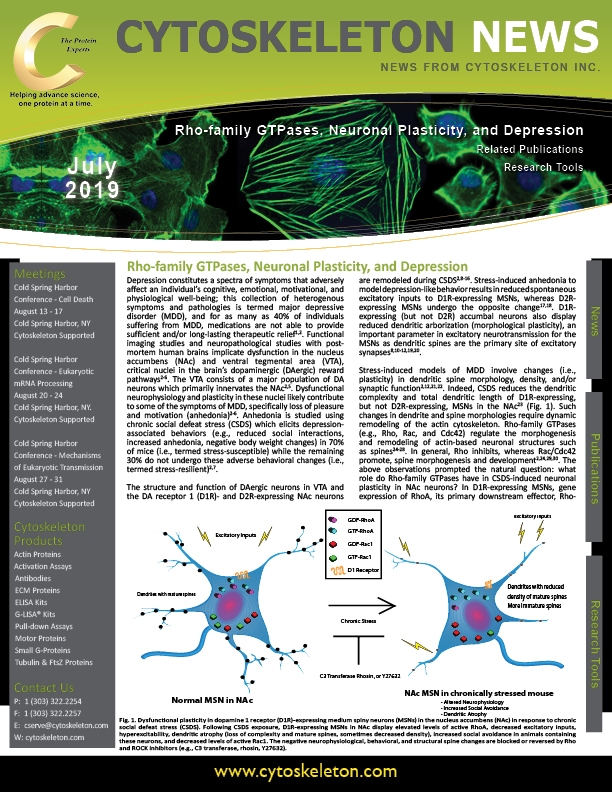
Depression constitutes a spectra of symptoms that adversely affect an individual’s cognitive, emotional, motivational, and physiological well-being; this collection of heterogenous symptoms and pathologies is termed major depressive disorder (MDD), and for as many as 40% of individuals suffering from MDD, medications are not able to provide sufficient and/or long-lasting therapeutic relief1,2. Functional imaging studies and neuropathological studies with post-mortem human brains implicate dysfunction in the nucleus accumbens (NAc) and ventral tegmental area (VTA), critical nuclei in the brain’s dopaminergic (DAergic) reward pathways2-6. The VTA consists of a major population of DA neurons which primarily innervates the NAc2,5. Dysfunctional neurophysiology and plasticity in these nuclei likely contribute to some of the symptoms of MDD, specifically loss of pleasure and motivation (anhedonia)2-6. Anhedonia is studied using chronic social defeat stress (CSDS) which elicits depression-associated behaviors (e.g., reduced social interactions, increased anhedonia, negative body weight changes) in 70% of mice (i.e., termed stress-susceptible) while the remaining 30% do not undergo these adverse behavioral changes (i.e., termed stress-resilient)2,7.
The structure and function of DAergic neurons in VTA and the DA receptor 1 (D1R)- and D2R-expressing NAc neurons are remodeled during CSDS2,8-16. Stress-induced anhedonia to model depression-like behavior results in reduced spontaneous excitatory inputs to D1R-expressing MSNs, whereas D2R-expressing MSNs undergo the opposite change17,18. D1R-expressing (but not D2R) accumbal neurons also display reduced dendritic arborization (morphological plasticity), an important parameter in excitatory neurotransmission for the MSNs as dendritic spines are the primary site of excitatory synapses8,10-12,19,20.
Also included in this newsletter:
- Tubulin and Actin Live Cell Reagents, G-LISA Activation Assay Kits, Tubulin Kits, Actin Biochem Kits
- Related Publications
Rab GTPases, members of the Ras super-family of GTPases, express at least 60 isoforms in humans1 with 24 enriched in, or specific for, the central nervous system (CNS)2. Rab GTPases are integral regulators of intracellular membrane trafficking, regulating the formation, maturation, transport, tethering, and fusion of vesicles in the endomembrane system3. In neurons, Rab-mediated membrane/vesicle trafficking is involved in virtually every aspect of neuron physiology, and dysfunction has been implicated in several neurodegenerative diseases2,4-7 (Fig. 1). Indeed, dysfunctional membrane trafficking is an early marker of neurodegeneration5. Here, Rab-mediated trafficking defects in Parkinson’s disease (PD) and Alzheimer’s disease (AD) are reviewed.
The cause of PD is primarily idiopathic – only a small fraction of PD cases are familial and attributable to mutations in proteins that also are involved in Rab activity and membrane trafficking. Mutant proteins include Rab39b, α-synuclein, PTEN-induced putative kinase (PINK1), and leucine-rich repeat kinase 2 (LRRK2)4,6,7. Rab39b is particularly interesting because loss-of-function mutations in this gene are directly linked to inherited early-onset PD with Lewy body pathology and also...
Also included in this newsletter:
- Signal-Seeker™ PTM Kits and Antibodies
- Related Publications
Ras GTPases regulate cell proliferation pathways, making them important molecules in oncogenesis and cancer cell migration and invasion. The four isoforms of Ras, H-Ras, N-Ras, K-Ras4A, and K-Ras4B (due to alternative splicing), were identified over 30 years ago for their oncogenic activation in human tumors. Activating Ras mutations are single amino acid substitutions (e.g., G12C, G12V, G12D) and have been identified in approximately 30% of all human cancers.
The same signaling pathways activate all Ras isoforms via guanine exchange factor (GEF)-mediated exchange of GDP for GTP, followed by binding to the same effector proteins. However, Ras oncogenic isoforms are differentially expressed aacross different cancers with oncogenic specificity significantly favoring K-Ras,. Indeed, K-Ras is the most common mutated Ras isoform (86% of all Ras mutations) and is correlated with over 21% of human cancers. In particular, K-Ras is the predominant or exclusive Ras gene mutated in three of the top four cancers with the highest mortality rates in the US: lung, colon, and pancreatic cancers. In most instances, the K-Ras4B is the primary isoform mutated in K-Ras-associated cancers. This newsletter discusses potential explanations for the biological basis of K-Ras’s oncogenic specificity.
Also included in this newsletter:
- Ras and GEF Proteins, Kits, and more
- Related Publications
Dendritic spines are the post-synaptic component of most excitatory glutamatergic synapses and primary site of synaptic structural plasticity for modulating synaptic function. Activity-dependent structural plasticity in spines (i.e., spine morphogenesis) depends upon dynamic re-organization of F-actin, the primary structural component of spines. Spine morphogenesis is important for normal learning and memory and the development of neurodegenerative diseases and neurological disorders.
The RhoA, Rac1, and Cdc42 GTPases regulate spine morphogenesis; RhoA inhibits spine growth and stability, whereas Rac1 and Cdc42 exert the opposite effect. In reality, Rho family regulation of spine structural plasticity is much more complex. Precise spatiotemporal regulation of Rho GTPases is with guanine exchange factors (GEFs) triggering GTP/GDP exchange and GTPase activating proteins (GAPs) stimulating intrinsic GTPase activity. At least eight Rho family GEFs regulate spine morphogenesis and these GEFs are activated through a variety of receptor signaling pathways, including glutamatergic NMDA receptors (NMDARs) and receptor tyrosine kinases (RTKs). NMDARs mediate calcium influx and subsequent activation of calcium/calmodulin-dependent kinases (CaMKs), which phosphorylate Rho family GEFs, essential for GEF activity. In this newsletter, the regulation of spine structural plasticity by the Rho family GEFs Kalirin7 (Kal7; the most abundant isoform in adult brain), Trio-9 (the most abundant isoform in hippocampus), Tiam1, RasGRF2, DOCK10, DOCK180, ephrexin1, and ephrexin5 is discussed.
Also included in this newsletter:
- GEF Proteins, Exhange Assays, Spirochrome Live Cell Imaging Probes, and more
- Related Publications
Intellectual disabilities (e.g., neurodevelopmental disorders, autism spectrum disorders [ASDs]) are associated with abnormal development of dendrites and dendritic spines. ASDs are a complex set of behaviorally defined disorders, characterized by impairments in social interaction, communication, and restricted or stereotyped behaviors. Recent studies estimate that 1% of the world-wide population has an ASD1. Dendritic spines are comprised of F-actin and the structural and functional plasticity of spines depend upon the dynamic regulation of actin by Rho-family GTPases. Indeed, Rac and PAK effector proteins are essential regulators of normal brain development and function, including dendritic spine initiation, elongation, and branching2-4. Recent genetic studies revealed that individuals with intellectual disabilities express mutated versions of genes involved in Rho-family GTPase signaling such as a Rho-family GTPase activating protein (GAP), the serine/threonine kinase PAK3, and the Rac/Cdc42 guanine exchange factor (GEF) aPIX5. In addition, the PAK inhibitor FRAX486 is an effective treatment for fragile X syndrome (FXS), the most common inherited form of autism and cognitive disability. FRAX486 reversed dendritic spine and behavioral abnormalities in an in vivo model of FXS6. Moreover, Rac1 activation or inhibition of cofilin, an actin depolymerizing protein, rescues ASD-like phenotypes in Shank3 knock-out mice, an in vivo model of ASDs7-12.
Also included in this newsletter:
- GTPase Assay Kits, Spirochrome™ Live Cell Imaging Probes, GEF Proteins, and more
- Related Publications
The small GTPase ADP-ribosylation factor 6 (Arf6) belongs to the Arf subfamily of Ras superfamily GTPases. Of the three classes of Arf GTPases, Arf6 is the only member of class III and uniquely localizes to the plasma membrane and endosomes, positioning it to regulate cellular processes dependent upon dynamic changes in the actin cytoskeleton, including endocytosis, exocytosis, trafficking/recycling of membrane-localized proteins, and membrane protrusions (e.g., ruffles). These cellular functions underlie physiological and pathological cell motility and intracellular trafficking. Arf6 cycles between an inactive, GDP-bound state and an active, GTP-bound state to act as a molecular switch in the cellular processes listed above. Activation of Arf6 by exchange of GDP for GTP is mediated by guanine exchange factors (GEFs) while inactivation by GTP hydrolysis is mediated by GTPase activating proteins (GAPs)1-3. In this newsletter, we discuss the mechanistic roles Arf6 and its GEFs have in cancer cell invasion and metastasis.
Click to read more
Also included in this newsletter:
- Arf Activation Assay Kits, F-actin Visualization Reagents, Other Live Cell Imaging Probes
- Related Publications
Ras and Rho-family GTPases regulate multiple cellular processes, including development, growth, motility, intracellular trafficking, gene expression, and the cell cycle1,2. Moreover, dysfunction of these GTPase are correlated with several human diseases (e.g., cancer, neurodegeneration, bacterial pathogenesis)3-5. Like all GTPases, Ras and Rho GTPases cycle between active (GTP-bound) and inactive (GDP-bound) states. Guanine nucleotide exchange factors (GEFs) regulate GTPase activation, driving the exchange of GDP for GTP in response to a variety of physiological and pathological extracellular signals1,2. Thus, GEFs are therapeutic targets; however, small molecule inhibitors require hydrophobic pockets for binding which are not typically found on GEFs (or GTPases). Only recently have novel binding pockets on GEFs and GTPases been discovered6,7. In this light, the current newsletter explores small molecule inhibitors of Ras (N-, H-, K-Ras) and Rho (RhoA, Rac1, Cdc42) GEFs that inhibit through direct binding.
Also included in this newsletter:
- Custom GEF Protein Production, G-protein Effector Proteins and Kits, G-Switch Activators and Inhibitors
- Related Publications
The Ras GTPase superfamily, which includes Ras, Rho, Rab, Arf, and Ran subfamilies (among others), has been shown to regulate a wide spectrum of cellular functions. GTPases function as molecular switches, cycling between an inactive GDP-bound form and an active GTP-bound form. The Rho GTPase subfamily includes Rho, Rac, and CDC42, and is believed to be involved primarily in the regulation of cytoskeletal organization in response to extracellular growth factors...
Also included in this newsletter:
- Antibodies, G-Switch Activators and Inhibitors, Activation Assays, G-protein Effector Proteins and Kits, NEW Signal Seeker Kits
- Related Publications
Elevations in blood glucose levels are sensed in pancreatic b-cells, which respond through a complex signaling pathway involving mitochondrial-dependent glucose metabolism1. The culmination of this pathway is the mobilization of intracellular insulin-loaded vesicles that fuse with the cell membrane releasing their contents into the...
Also included in this newsletter:
- Rac1 Activation Assays, Antibodies, Custom GEF Screenings and New Signal Seeker Kits
- Related Publications
Cytoskeleton, Inc. was founded in 1993 and coincidentally that was the era when the Rho family of small G-proteins was determined to be major regulators of cytoskeletal dynamics. This finding was established in a very direct way by the seminal paper authored by Drs. Anne Ridley and Alan Hall who at that time worked at the Medical Research Council in London(1). Since then,Click to read more
Also included in this newsletter:
- Related Publications
The Ras GTPase plays an important role in multiple signal transduction pathways involved in normal cell growth and differentiation as well as several forms of cancer1,2. The three isoforms of Ras, H-Ras, N-Ras, and K-Ras, were identified over 30 years ago for their oncogenic activation in human tumors1,2. Aberrant Ras signaling has been identified in...Click to read more
Also included in this newsletter:
- Available Research Tools and Services
- Related Publications
YAP1 and the Hippo Pathway
The Hippo signal transduction pathway plays a critical role in the regulation of organ size through the coordinated modulation of cell fate(1). The core pathway memebers include two sets of serine/threonine kinases (MST1/2 and LATS1.2) that act...Click to read more
Also included in this newsletter:
- Available Research Tools and Services
- Related Publications
The activity of Rho family GTPases is regulated temporally and spatially by a variety of direct post-translational modifications (PTMs) that include prenylation, ubiquitination, oxidation, nitrosylation, and phosphorylation (Fig. 1). This newsletter focuses on control of RhoA function through phosphorylation. RhoA is a target for a growing number of kinases and as such, phosphorylation is emerging as a central theme in the regulation of this family of proteins2... Click to read more
Also included in this newsletter:
- Available Research Tools and Services
- Related Publications
Ras and Rho family GTPases are cytoskeletal small G-proteins that critically regulate multiple actin-dependent cell processes, including development, growth, motility, and intracellular trafficking1,2. Moreover, dysfunction of Ras and Rho family GTPases are correlated with several human diseases (e.g., cancer, neurodegeneration) and these GTPases are... Click to read more
Also included in this newsletter:
- Available Research Tools and Services
- Related Publications
K-Ras has been identified as the most important Ras protein in cancer research, accounting for over 21% of human cancers. Despite extensive research on these proteins, no effective Ras inhibitor has been identified, earning K-Ras the reputation of an undruggable protein... Here we discuss several leading concepts in the pursuit of identifying viable drug targets to control atypical Ras signaling... Click to read more
Also included in this newsletter:
- Small GTPase Activation Assays, Activators, Inhibitors, Proteins and more.
- Related Publications
- Related Research Tools and Services
Redox agents, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), are key regulators in a variety of signal transduction pathways, including integrin signaling, extracellular matrix adhesion, and inflammation1-3. Rho GTPases are also key regulators of many cellular processes, including cell growth, motility, and adhesion4. While redox agents and Rho GTPases operate through a wide array of... Click to read more
Also included in this newsletter:
- Small GTPase Activation Assays, Activators, and Inhibitors
- Related Publications
- Related Research Tools and Services
Ras and Rho GTPases are small G-proteins that cycle between an active GTP-bound form and inactive GDP-bound form. Ras proteins, known for their role in cell proliferation, and Rho proteins, known for their involvement in cell morphology, have common post-translational modifications (PTMs) that have been identified as contributors to oncogenesis1,2. Understanding Ras and Rho PTMs have been of interest for drug discovery groups for many years. Recent studies of signaling pathways mediated by the Ras and Rho PTMs prenylation and/or palmitoylation have identified potential cancer drug targets1,2... Click to read more
Also included in this newsletter:
- Ras and Rho Related Publications
- Ras and Rho Protein Research Tools
This month, the focus is on dendritic spine and the Arf6. This months newsletter features the following:
- Dendritic Spines: Role of Arf6 in Development
- Arf Related Publications
- Arf Protein Research Tools
Click to download our January Newsletter.
This month, the focus is on Ubiquitination and the Regulation of Rho Family Pathways. This months newsletter features the following:
- Ubiquitination and the Regulation of Rho Family Pathways
- Rho Ubiquitination Publications
- Rho Family Research Tools
Click to download our October Newsletter.
This month, the focus is on Rac1 GTPase and Neurodegeneration. This months newsletter features the following:
- The Role of Rac1 GTPase in Neurodegeneration
- Rac1 GTPase and Neurodegeneration Publications
- Rho Family Research Tools
This month, the focus is on Epithelial-Mesenchymal Transition (EMT) and the Involvement of Rho Family Small G-proteins. This months newsletter features the following:
- Epithelial-Mesenchymal Transition (EMT) and the Involvement of Rho Family Small G-proteins
- EMT Publication Spotlight
- Rho Family Research Tools
Click to download our August Newsletter.
This month's newsletter focus is on Arf protein research. In this issue you will find useful information on the following topics:
- Trafficking: Arfs and the Cdc42/Rac connection
- Arf Protein Publications
- Arf Protein Tools
Click to download our March Newsletter.
We are pleased to introduce two new activators to our G-switch™ product line. Based on the bacterial CNF toxins, these activators selectively target either Rho (Cat. # CN03) or Rho/Rac/Cdc42 combined (Cat.# CN04). Features of these new reagents include:
- Potent activation (5 to 10 fold) of Rho family members.
- Effective in multiple cell types derived from different species.
- Direct activation within 2 to 4 hours.
Click to download our September Newsletter.
This month newsletters focus is on Small G-protein Activation Assay
In this issue you will find useful information on the following topics:
- Measuring Active Small G-Proteins
- Publication Spotlight
- RhoA G-LISA Activation Assay
- Wide variety of G-LISA Products