Myosin protein: bovine cardiac muscle
Product Uses Include
- Drug discovery target in cardiac research
- Measurement of F-actin activated myosin ATPase activity
- Identification/characterization of proteins or small molecules that affect myosin II ATPase activity
- Identification/characterization of proteins or small molecules that affect the myosin II - F-actin interaction
- Control myosin ATPase activity in drug discovery screening projects
Material
Cardiac myosin protein has been purified from bovine heart muscle. The full length myosin protein has been purified with its essential light chains (ELC) and regulatory light chains (RLC), see Figure 1 and 2. Cardiac myosin has been determined to be biologically active in an F-actin activated ATPase assay, but it is not recommended for use in motility assays because of auto-inhibition (Protease digests with alpha-chymotrypsin and papain may create a subfragment that has F-actin moving properties. Cardiac myosin protein is supplied as a white lyophilized powder. When reconstituted in nanopure water, the protein will be in the following buffer: 100
mM Imidizole pH 7.5, 2.4 M KCl, 0.2 mM DTT, 5% sucrose and 1% dextran. The lyophilized protein is stable at 4°C desiccated (<10% humidity) for 1 year.
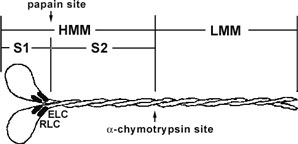
Figure 1. Diagrammatic representation of the myosin II protein and its subfragments. Myosin II or conventional myosin is a hexameric protein consisting of two heavy chains and two light chains. Myosin II can be proteolytically cleaved into heavy meromyosin (HMM, Cat.# MH01) and light meromyosin (LMM) by α-chymotrypsin. Heavy meromyosin consists of the myosin head subfragment-1 domain (S1), its associated light chains (essential light chains and regulatory light chains), and the coiled-coil subfragment -2 domain. Light meromyosin consists of coiledcoil protein structure. The myosin S1-subfragment is produced by papain digestion of HMM.
Purity
Protein purity is determined by scanning densitometry of Coomassie Blue stained protein on a 4-20% gradient polyacrylamide gel. Myosin protein is 95% pure (see Figure 2).
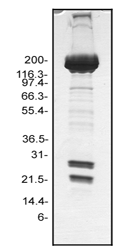
Figure 2. Myosin protein purity determination. A 20 µg sample of MY03 was separated by electrophoresis in a 4-20% SDS-PAGE system, and stained with Coomassie Blue. The myosin heavy chain is located at approximately 200 kDa, whereas the RLC is approximately 20 kDa and the ELC isoform is approximately 25 kDa). Protein quantitation was performed using the Precision Red Protein Assay Reagent (Cat.# ADV02).
Biological Activity
The biological activity of cardiac myosin is determined from its rate of F-actin activated ATP hydrolysis. A standard biological assay for monitoring ATP hydrolysis by myosin consists of an in vitro F-actin ATPase assay (Cat. # BK054). Stringent quality control ensures that in the presence of F-actin, rabbit myosin will have a minimum hydrolysis rate 10 fold greater than in the absence of F-actin, which is comparable to published results. Rabbit myosin (Cat.# MY02) is also available for comparison studies.
For product Datasheets and MSDSs please click on the PDF links below. For additional information, click on the FAQs tab above or contact our Technical Support department at tservice@cytoskeleton.com
Question 1: Does this myosin contain both light and heavy chains and all other subunits?
Answer 1: Yes, this protein is full length myosin motor protein isolated from bovine cardiac muscle. The myosin protein contains the two heavy chains and two light chains, the essential light chain (ELC) and regulatory light chain (RLC).
Question 2: Does this myosin have ATPase activity that is activated by actin filaments?
Answer 2: Yes, the biological activity test for the myosin protein is in vitro measurement of myosin’s ATPase activity in the activating presence of actin filaments. Stringent quality control ensures that in the presence of F-actin, bovine cardiac myosin will have a minimum hydrolysis rate 2 fold greater than in the absence of F-actin. This myosin has approximately 20 times less ATPase activity than skeletal muscle myosin.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com