Profilin 1 protein: Untagged, human recombinant
Product Uses Include
- Actin binding studies
- Actin polymerization studies
Material
Profilin protein has been expressed in E.coli via chromatography without a tag. Thus the protein does not contian a 6xHis or other tag. The protein is supplied as a lyophilized powder. When resuspended in nanopure water, the profilin will be in the following buffer: 10 mM Tris pH 8.0, 1 mM EDTA, 1 mM DTT, 5% (w/v) sucrose and 1% (w/v) dextran.
The lyophilized protein is stable at 4°C desiccated (<10% humidity) for up to 1 year.
Purity
Purity is determined by scanning densitometry of proteins on SDS-PAGE gels. Purity is >95% (see figure 1).
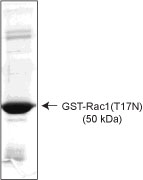
Figure 1. Profilin purity determination. A 10 µg sample of PR02 (profilin molecular weight approx. 15 kDa) was run on an SDS-PAGE gel and stained with coomassie blue. Protein quantitation was performed using the Precision Red Protein Assay Reagent (Cat.# ADV02).
Biological activity
Profilin is tested for biological activity by a binding assay with actin. Profilin is bound to poly-L-proline sepharose beads and incubated with G-actin. The profilin beads bind >90% of the available actin by this procedure.
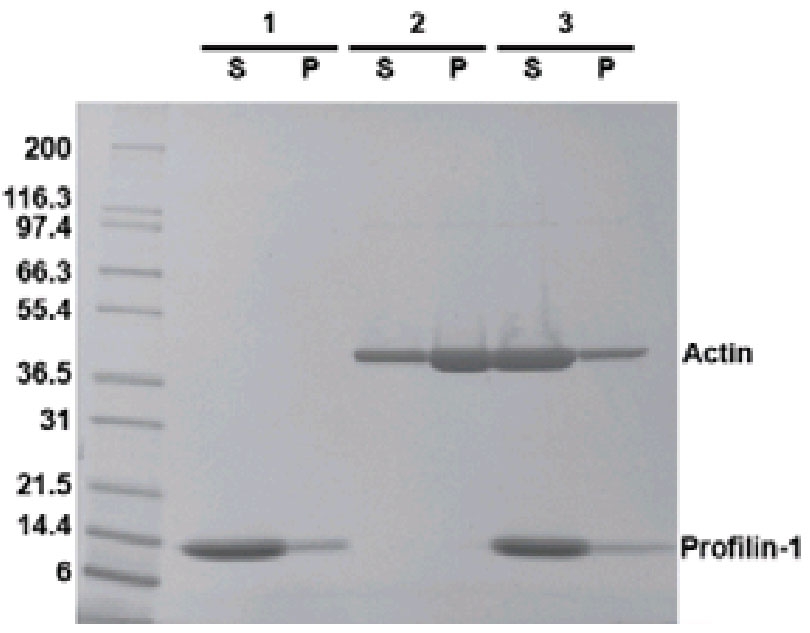
Actin Polymerization Inhibition Assay. The ability of profilin to inhibit actin polymerization was assessed by SDS-PAGE of proportionally loaded supernatant (S) and pellet (P) fractions from G-actin incubated with and without profilin-1 according to the assay method. In the absence of profilin-1, approx. 80% of the actin protein (43kDa) is found in the pellet fraction as F-actin (P, lane 2). When G-actin is incubated with profilin prior to polymerization, only 20% approx. of actin is found as F-actin in the pellet (P, lane 3), while the other 80% remains as G-actin in the supernatant (S, lane 3). Lane 1, profilin-1 protein alone. Mark12 molecular weight markers are from Invitrogen.
Kinetic assay using profilin to inhibit Arp2/3 induced polymerization
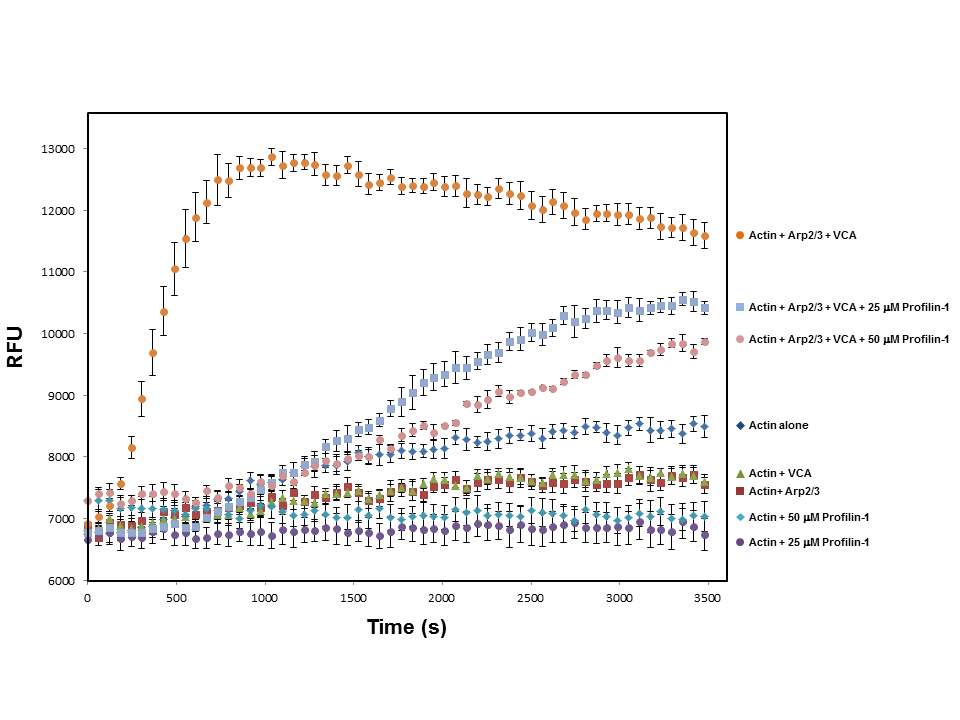
Profilin-1 Inhibits Branched Polymerization of Actin Filaments by the Arp2/3 Complex and the VCA Domain of WASP. Actin polymerization was carried out as described in the method; all reactions contain a 1:1 ratio of pyrene- and nonlabeled-actin. Actin in the presence of both Arp2/3 and VCA shows enhancement of actin nucleation. Upon the addition of Profilin-1, the steep nucleation phase provided by both Arp2/3 and VCA is delayed and greatly reduced. The additionof Profilin-1 to actin also decreases the rate of actin polymerization when compared to actin alone.
For product Datasheets and MSDSs please click on the PDF links below. For additional information, click on the FAQs tab above or contact our Technical Support department at tservice@cytoskeleton.com
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com