Cdc42 G-LISA Activation Assay (Colorimetric format) - 24 assays
G-LISA Cdc42 Activation Assay Biochem Kit (Colorimetric format)
Product Uses Include
- Rho signaling pathway studies
- Rho activation assays with primary cells
- Rho activation assays with limited material
- High throughput screens for Rho activation
Introduction
The G-LISA® series of Small G-Protein Activation Assays are ELISA based assays with which you can measure the GTP form of small G-proteins from lysates of cells or tissues and all in less than 3 h. The Cdc42 G-LISA Activation Assay measures the entire level of GTP-loaded Cdc42 protein in cell lysates. The level of activation is measured by reading at OD490nm. For a more detailed introduction on G-LISA assays and a listing of other available G-LISA kits, see our main G-LISA page. For a kit to measure RhoA activation please check webpage BK124, for Rac1 go to BK128 for Rac1,2,3 go to BK125, for RalA go to BK129.
The Cdc42 G-LISA Activation Assay is very sensitive and has excellent accuracy for duplicate samples. See this section on our G-LISA information resource page.
Example results
Serum starved Swiss 3T3 cells were stimulated with the Cdc42 activating compound EGF and Cdc42 activation was measured with the G-LISA method (Fig 1 and 2).
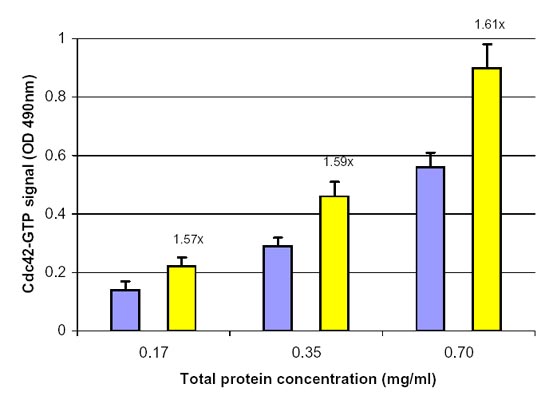
Figure 1. Cdc42 activation by EGF measured by the Cdc42 G-LISA Activation Assay. Swiss 3T3 cells were serum starved (SS) for 16 h at 1% serum and 8 h with 0% serum and treated with EGF (100 ng/ml for 2 min). Cell lysates (8, 17, 35 µg) were subjected to the G-LISA assay. Data was read at 490 nm. Numbers on top the yellow columns indicate the fold increase in signal caused by EGF activation, you will notice the ratio remains the same with different protein loadings indicating good linearity with different protein loadings. 500 µg of the same lysates were subjected to the traditional PAK pull-down assay (Cat.# BK034) with similar results.
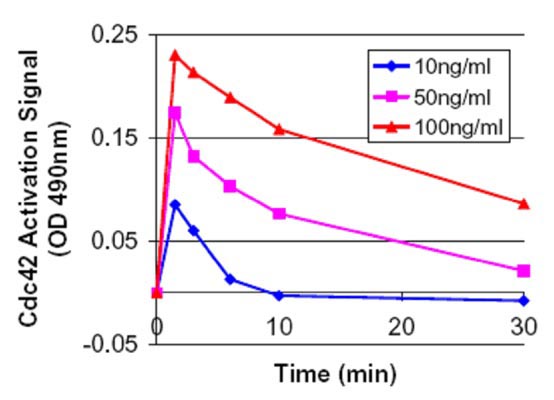
Figure 2. Time course of Cdc42 activation using EGF at 10, 50 and 100ng/ml . Swiss 3T3 cells were serum starved (SS) for 16 h at 1% serum and 8 h with 0% serum and treated with EGF (10, 50 and 100 ng/ml for1.5, 3.0, 6.0, 10 and 30 min). Cell lysates subjected to the G-LISA™ assay and OD was read at 490 nm. The “controlled state” serum starved value (0.22) was subtracted from these samples prior to plotting. At 100 ng/ml the total activation was 2.1 fold or 110% over the controlled state at 1.5 min. This type of analysis can be performed in one afternoon.
Kit contents
The kit contains sufficient reagents to perform 24 Cdc42 activation assays. Since the Cdc42-GTP affinity wells are supplied as strips and the strips can be broken into smaller pieces, each kit can be used for anywhere from 1 to 96 assays. The following components are included in the kit:
- Cdc42-GTP affinity wells (3 strips of 8 wells each)
- Lysis buffer
- Antigen presenting buffer
- Wash buffer
- Antibody dilution buffer
- Anti-Cdc42 antibody (Cat.# ACD03)
- HRP-labeled secondary antibody
- Positive control Cdc42 protein
- Protease inhibitor cocktail (Cat. # PIC02)
- Color development reagents
- Precision Red™ Advanced protein assay reagent (Cat. # ADV02)
- Manual with detailed protocols and extensive troubleshooting guide
Equipment needed
- 96-well plate spectrophotometer capable of reading 490 nm wavelength
- Multichannel or multidispensing pipettor (see Biohit pipettors)
- Two orbital microplate shakers capable of 400 rpm shaking (200 to 400 rpm is the possible range)
Examples are:
Model # 4625 Titer Plate Shaker, Lab-Line Instruments, Barnstead Intl.(average priced)
Model # RF7854 Digital Microplate Shaker, ML Market Lab, researchml.com (economical priced)
Model # RF7855 Incubating Microplate Shaker, ML Market Lab, researchml.com (deluxe model)
G-LISA Products:
Rac1,2,3 G-LISA Activation Assay, colorimetric format (Cat.# BK125)
Rac1 G-LISA Activation Assay, luminescence format (Cat.# BK126)
RhoA G-LISA Activation Assay, colorimetric format (Cat.# BK124)
RhoA G-LISA Activation Assay, luminescence format (Cat.# BK121)
Associated Products:
Anti-Cdc42 monoclonal antibody (Cat.# ACD03)
Anti-Rac1 monoclonal antibody (Cat.# ARC03)
Anti-RhoA monoclonal antibody (Cat.# ARH03)
G-LISA is a registered trademark of Cytoskeleton, Inc (CO). All rights reserved.
For product Datasheets and MSDSs please click on the PDF links below.
 G-LISA Activation Assay Technical Guide download here
G-LISA Activation Assay Technical Guide download here G-LISA Data Analysis (Absorbance) Excel Template download here.
G-LISA Data Analysis (Absorbance) Excel Template download here.
Question 1: Can I use the lysis buffer from the Cdc42 G-LISA activation assay kit (Cat. # BK127) to prepare samples for the other G-LISA assays?
Answer 1: The Rac1 and RhoA G-LISAs use the same lysis buffer (Part # GL36). The Cdc42 G-LISA kit (Cat. # BK127) uses a different lysis buffer (Part # GL35). The buffer components are proprietary, but in general, the lysis buffers contain a buffer, detergents and salts. GL35 is about 2X more concentrated than GL36 in regard to salt and detergent concentrations. So you could make the extracts in GL35 and dilute them in GL36 for the Rac1 and RhoA assays. As a reminder, be sure to aim for approximately 0.5-1 mg/ml protein concentration when performing the lysis. At higher concentrations, you are likely to have significant loss of signal due to proteolysis, increased phosphatase/kinase activity and increased GAP activity.
Question 2: How many cell culture plates can I process at one time during the lysis step?
Answer 2: We recommend that from the point at you add lysis buffer to the plate on ice to aliquoting and snap-freezing the lysate samples in liquid nitrogen, no more than 10 min are allowed to elapse. After 10 min on ice, we find that GTP bound to GTPases (activated GTPases) undergoes rapid hydrolysis. Rapid processing at 4°C is essential for accurate and reproducible results. The following guidelines are useful for rapid washing of cells.
Washing
a. Retrieve culture dish from incubator, immediately aspirate out all of the media and place firmly on ice.
b. Immediately rinse cells with an appropriate volume of ice cold PBS (for Cdc42 activation, skip this step and simply aspirate the media) to remove serum proteins.
c. Aspirate off all residual PBS buffer. This is essential so that the Lysis Buffer is not diluted. Correct aspiration requires that the culture dish is placed at a steep angle on ice for 1 min to allow excess PBS to collect in the vessel for complete removal. As noted, the time period between cell lysis and addition of lysates to the wells is critically important. Take the following precautions:
1. Work quickly.
2. Keeping solutions and lysates embedded in ice so that the temperature is below 4°C. This helps to minimize changes in signal over time.
3. We strongly recommend that cell lysates be immediately frozen after harvest and clarification. A sample of at least 20 μl should be kept on ice for protein concentration measurement. The lysates must be snap frozen in liquid nitrogen and stored at -70°C. Lysates should be stored at -70°C for no longer than 30 days.
4. Thawing of cell lysates prior to use in the G-LISA assay should be in a room temperature water bath, followed by rapid transfer to ice and immediate use in the assay.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com.





