Oncogenic RAS signaling and Tumor Immune Evasion: Mechanisms and Therapeutic Opportunity
Oncogenic RAS drives cells to the cancerous state through a wide range of mechanisms, including: the establishment of cancer cell plasticity (1), enhancement of cell migration and metastasis (2) and metabolic reprogramming towards anabolic metabolism (3). This Newsletter focuses on the emerging area of RAS driven oncogenesis via the evasion of host immune attack.
Cancerous cells are visible to the host immune system through chronic stress signals and tumor associated antigen presentation and are therefore susceptible to being cleared from the host via immunogenic mechanisms. Many RAS driven cancers have a high mutational burden and should be good targets for immune clearance, however, oncogenic RAS has evolved mechanisms to suppress the immune response and promote tumor growth. A brief overview of the major mechanisms is summarized below.
Enhanced Immune Checkpoint Protein Expression
Engagement of cytotoxic T-cells with PD-L1, results in T cell exhaustion and a decrease in immune cell tumor infiltration (4). Oncogenic RAS signaling promotes cancer cell immune evasion through increased expression of the transmembrane immune checkpoint protein PD-L1. Regulation occurs at the transcriptional and post transcriptional level. For example, oncogenic KRAS causes stabilization of PD-L1 mRNA mediated through three AU rich elements (AREs) in the 3’ untranslated region (UTR) of PD-L1 mRNA (5). In non-transformed cells an ARE binding protein (AUBP), tristeraprolin (TTP), binds to PD-L1 mRNA and recruits mRNA decay proteins, resulting in a short (1h) half-life of PD-L1 mRNA. Oncogenic RAS inhibits TTP activity through a RAS-MEK-ERK-p38-MK2 axis that is activated through oncogenic RAS/MEK driven reactive oxygen species (ROS) generation. ROS accumulation causes the stress kinases p38 and MK2 to inactivate TTP through serine phosphorylation (Fig 1). Enhanced PD-L1 expression through KRAS has also been reported to occur via a MAPK-dependent transcriptional activity of AP-1 and STAT-3 (6).
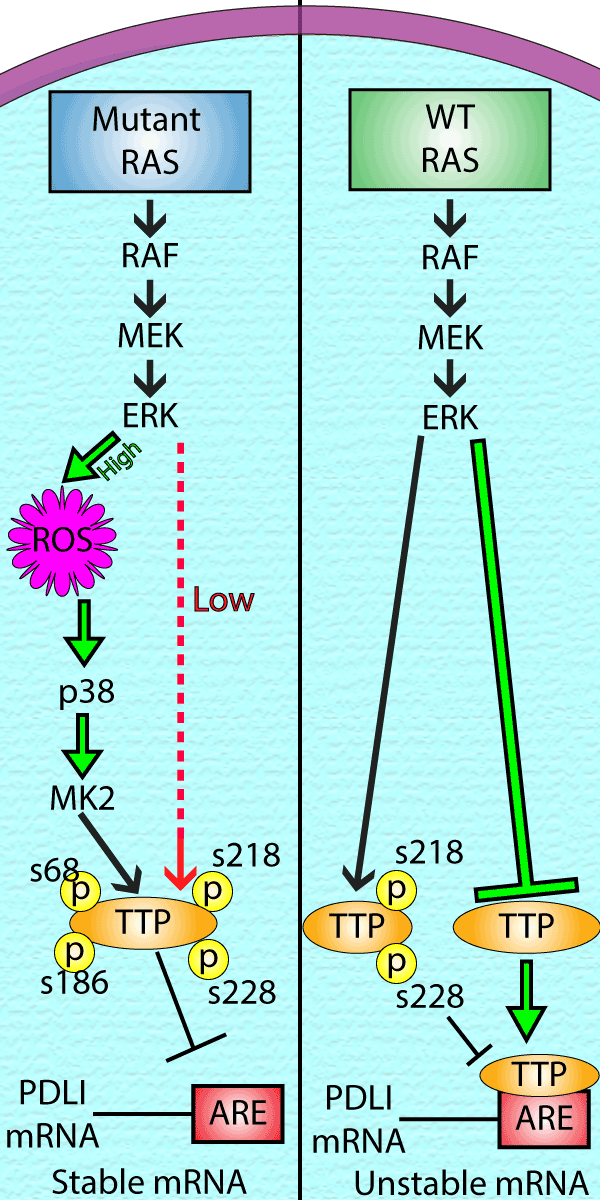
Fig.1 Downstream signaling of Mutant and WT Ras and the downstream effects result in Stable vs Unstable mRNA
Suppression of Major Histocompatibility Complex (MHC) Class I Expression
MHC-1 presents tumor derived peptides to cytotoxic T cells leading to cell destruction, disruption of this process renders tumor cells resistant to the immune system (7). Oncogenic RAS signaling has been implicated in reducing the expression of genes involved in the presentation of tumor neo-antigens and other tumor associated antigens by MHC class I (8). The mechanisms involved in reduced MHC-1 expression include a RAS driven insensitivity to interferon gamma (IFNƴ) signaling through reduced expression of the IFN responsive transcription factors STAT1, STAT2 and IRF-9 (9,10). Mutant KRAS attenuation of MHC-1/INF/STAT1 regulation has been confirmed in cell lines expressing inducible oncogenic KRASG12V. KRASG12V induction resulted in a significant decrease in antigen processing and presenting proteins such as TAP1, TAPBP, HLA-A and B2M which are regulated by STAT1 (5).
Modulation of the Tumor Microenvironment (TME)
The TME is composed of many cell types, including immune cells such as macrophages, myeloid derived suppressor cells (MDSCs), T- and B-cells and stromal cells such as fibroblasts and endothelial cells. Oncogenic RAS can alter the expression of many cytokines, chemokines, and growth factors to support a pro-tumor microenvironment (TME) and the suppression of anti-tumor immunity (11). For example, Theiventhian et al demonstrated that induction of the inflammasome complex NLRP3 was responsible for PD-L1 immunotherapy resistance via expansion of immunosuppressive MDSCs (12). Hamarsheh et al subsequently showed a direct link between oncogenic RAS and NLRP3 induction through interleukin ILK-1 signaling (13). RAS has also been demonstrated to support the accumulation of MDSCs through induction of the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) (14) and the proliferation of immunosuppressive Regulatory T cells (Tregs) through interferon and interleukin signaling (15,16).
Therapeutic Opportunities
Checkpoint immunotherapy targeting the CTLA-4 and PD-1/PD-L1 pathways have shown great promise clinically. Likewise, the newly FDA approved oncogenic KRAS G12C inhibitor, sotorasib, represents exciting progress for RAS targeted therapies. However, there is still a need to improve the moderate success rate and address adverse side effects and drug resistance. The link between oncogenic RAS and immune evasion suggests a combinatorial approach to achieve more efficacious cancer therapy. In this regard studies of combinatorial therapy in a mouse model of colorectal cancer resulted in enhanced T-cell tumor infiltration and increased tumor destruction (17). There are currently several ongoing clinical trials addressing this combinatorial strategy (18).
References
- Siyuan Q. et al. 2020. Emerging role of tumor cell plasticity in modifying therapeutic response. Nature: Sig. Trans. Targ. Ther. 5:228
- Gimple R.C & Wang X. 2019. RAS: Striking at the core of the oncogenic circuitry. Oncol. 9:965
- Tarrado-Castellarnau M. et al. 2016. Oncogenic regulation of tumor metabolic reprogramming. Oncotarget 7:62726-62753
- Loi S. et al. 2016. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple negative breast cancer: therapeutic cooperation between MEK and PD-L1 immune checkpoint inhibitors. Cancer Res. 22:1499-1509
- Coelho M.A. et al.2017. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Cell Immunity 47:1083-1099
- Sumimoto H. et al. 2016. RAS-mitogen-activated protein kinase signal is required for enhanced PD-L1 expression in human lung cancers. PLoS One 11:e0166626
- Jongsma M.L.M et al. 2021. Playing hide and seek: tumor cells in control of MHC class 1 antigen presentation. Immunol. 136:36-44
- Ebert, P.J.R et al. 2016. MAP kinase inhibition promotes T cell and anti-tumor-activity in combination with PD-L1 checkpoint blockade. Immunity 44:609-621
- El-Jawhari J.J. et al. 2014. Blocking oncogenic RAS enhances tumor cell surface MHC class I expression but does not alter susceptibility to cytotoxic lymphocytes. Immunol: 58:160-168
- Klampfer L. et al. 2003. Oncogenic Ki-ras inhibits the expression of interferon-responsive genes through inhibition of STAT1 and STAT2 expression. Biol. Chem. 278:46278-46287
- Hamarsheh S. et al. 2020. Immune modulatory effects of oncogenic KRAS in cancer. Comm. 11:5439
- Theivanthiran, B. et al. 2020. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti–PD-1 immunotherapy. Clin. Invest.130, 2570–2586
- Hamarsheh, S. et al. Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation. Commun.11, 1659 (2020)
- Pylayeva-Gupta Y. et al. 2012. Oncogenic KRAS-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21:836-847
- Kalvala A. et al. 2019. Phenotypic switching of native T-cells to immune-suppressive Treg-like cells by mutant KRAS. Clin. Med. 8:1726
- Zdanov S. et al. 2016. Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol. Res. 4:354-365
- Canon J. et al. 2019. The clinical KRAS (G12C) inhibitor AMG510 drives anti-tumor immunity. Nature 575:217-223
- Fakih M. et al. 2020. Trial in progress: a phase Ib study of AMG 510, a specific and irreversible KRAS G12C inhibitor, in combination with other anti-cancer therapies in patients with advanced solid tumors harboring KRAS pG12C mutation. Clin. Oncol. 38

