August 2020 Newsletter: Fluorescent Membrane Probes
Fluorescent Membrane Probes
This newsletter describes the pros and cons of commonly used fluorescent membrane probes and provides an overview of the latest probe breakthroughs. Luminescent membrane probes delineate the periphery of a cell by binding close to or within the plasma membrane. There are, broadly speaking, five classes of membrane probes, which are; wheat germ agglutinin (WGA) conjugates, lipid conjugates, lipophilic dyes, covalent binding probes, and biophysical reporter probes. Studies in the 1980s aimed at elucidating neuronal development, where neuronal morphology is paramount, used horseradish peroxidase (HRP)-conjugated WGA1 in fixed cells. However, the reaction required to visualize HRP was cytotoxic to cells which prohibited its use on living cells2. In 1986, Honig et al. demonstrated the paradigm shifting utility of a cationic carbocyanine dye by employing both 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiIC18) and 3,3´-dioctadecyloxacarbocyanine perchlorate (DiOC18), to visualize and identify several classes of live neurons in culture2. In 1989, Paul Karl Horan, then at Zynaxis Cell Science, developed a novel kit that expanded the utility of cyanine dyes via the addition of an osmolarity regulating agent and protocol that was designed to increase cell staining consistency and viability. In the early 2000s, WGA-HRP was supplanted by less-toxic fluorescently-conjugated WGA but still lacked the stability and efficiency required for long-term neuronal development studies1–3. Recently, new fluorogenic probes have been developed including MemGlow™ (MemBright™ family4), Flipper-TR5a and Nile Red-based probes5b,6, which bestow researchers unprecedented resolution of membrane structure and function, and with the latter two acting as biophysical sensors.
WGA
WGA is a lectin that preferentially binds cell surface glycans N-acetylglucosamine and N-acetylneuraminic acid7. These carbohydrates are commonly attached to proteins and their localization can produce indirect visualization of a cell’s membrane. Contemporary WGA markers now utilize Alexa Fluor-conjugation that permits the localization of glycan cell-surface markers enabling scientists to indirectly detect the plasma membrane. A significant downside of all fluorophore-conjugated WGA probes is that surface glycans can vary by composition of the cell membrane resulting in intercellular staining differences across a given cell population4,8. Moreover, labelled WGA cannot efficiently stain 3D cell culture and tissues due to their large size that limits their diffusion.
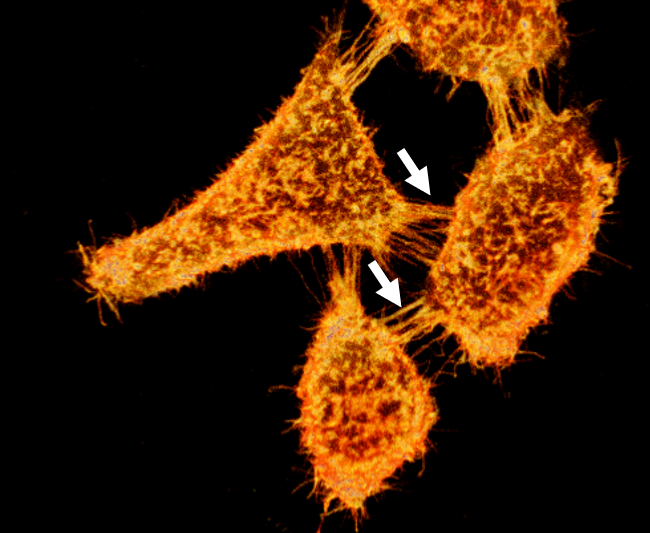
Widefield fluorescent imaging of live HEK293 labeled with MemGlow™ 488. Arrows indicate filopodia and nanotubular channels connecting cells. Image provided courtesy of M. Collot, CNRS, France.
Click Here for a PDF of this Newsletter
PKH
PKH dyes possess carbon chains that insert and anchor the fluorophore to the lipid regions of plasma membranes. PKH dyes permit the characterization of the plasma membrane in live cells with reported half-lives ranging from 5-100 days but requires a time-consuming protocol and requisite proprietary iso-osmotic reagent that reduces heterogenous staining. The chemistry behind cyanine Paul Karl Horan (PKH) dyes enables the specific labeling of the plasma membrane without the need for membrane-expressed factors. Currently, the available PKH dyes, comprised of PKH2, PKH26, and PKH67, have a limited emission spectra ranging from 504 nm to 567 nm. Following dye intercalation, the protocol requires dye quenching steps via the addition of serum or BSA proteins9,10. Failure to quench unbound dye can result in dye aggregation and carry over into co-cultures or cause cells to clump. Concerningly, emergent data suggest that labeling with PKH26 causes exosome swelling, which may alter exosome biodistribution and cellular uptake, confounding investigator results11. Generally, PKH labeling is inefficient due to poor water solubility require relatively high concentrations for effective membrane staining12.
Long-chain carbocyanine dyes
Long-chain lipophilic carbocyanine dyes, DiI, DiD, DiA, DiR, and DiO are well known plasma membrane dyes and retrograde neuronal tracers that cover a wide spectrum of excitation emissions. 3,3´-dioctadecyloxacarbocyanine perchlorate (´DiO´; DiOC18(3)) exhibits peak fluorescence at 501 nm, DiI peaks at 565 nm, 4-(4-(didecylamino)styryl)-N-methylpyridinium iodide (4-Di-10-ASP) peaks at 590 nm, 1,1´-dioctadecyl-3,3,3´,3´-tetramethylindodicarbocyanine perchlorate ('DiD' oil; DiIC18(5) oil) peaks at 665 nm, and 1,1´-dioctadecyl-3,3,3´,3´-tetramethylindotricarbocyanine iodide ('DiR'; DiIC18(7)) peaks at 780 nm. Although these carbocyanine dyes appear to broadly cover the visible spectral range, they are practically insoluble in water. Therefore, they strongly aggregate in aqueous solutions producing poorly controlled cell membrane staining12. For example, DiO fails to stain tissues in situations where DiA succeeds13, and DiI may label the membrane efficiently; however, far-red DiD produces punctate staining13. Carbocyanine dyes are also incompatible in co-labeling applications where Triton-X 100 is required to increase cell permeabilization4,14.
Advanced Membrane Probes
New membrane probes deserve special recognition as they provide microscopists with high resolution capabilities previously unattainable.
MemGlow™
The MemGlow™ (MemBright™ family) probes offer a new solution for staining phospholipid membranes and consist of 5 fluorogenic cyanine-based plasma membrane probes with a wide spectral range that includes near-infrared (Abs.max 499-688 nm, Em.max 506-713)4. The MemGlow™ product line possesses many of the desirable characteristics of their carbocyanine parent dyes, while exhibiting greater efficiency than popular labeling products in cellular applications. The advantages conferred to MemGlow™ dyes are owed to their chemical structures, based on a symmetrical cyanine enhanced by the chemical attachment of two zwitterionic anchors. Amphiphilic anchors improve water solubility in form of micellar aggregates and promote the dissociation and intercalation of probe aggregates into plasma membranes while simultaneously enhancing their retention (Figure 1). The red-shifted MemGlow™ probes (MemGlow™ 590 and MemGlow™ 700) possess additional fused benzene rings which confer increased lipophilicity and resistance to endocytosis.
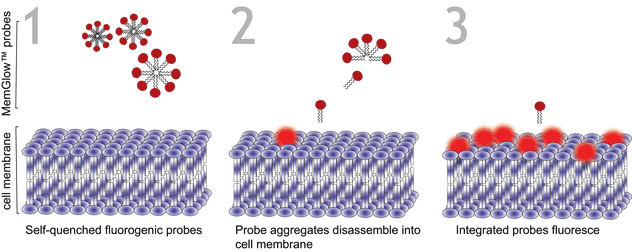
Figure 1. Turn-on mechanism of MemGlow™ probes. MemGlow™ probes are self-quenched nanoparticles until integration with the plasma membrane enables their excitation.
MemGlow™ application is simple. When MemGlow™ probes are introduced into aqueous media the amphiphilic probes form self-quenching aggregates until contact with a plasma membrane elicits their dissociation and dispersal into the lipid bilayer. Once integrated, the fluorogenic probes are ready for bioimaging. From MemGlow™ 488 to MemGlow™ 700, MemGlow™ probes produce intense fluorescence many times greater than background and exhibit good signal-to-background ratios4. MemGlow™ dyes are a technological breakthrough and superior to many contemporary stains (Table 1) such as: 1) PKH dyes which require a convoluted protocol and alter normal biology , 2) long-chain carbocyanine dyes that aggregate strongly and thus possess variable non-uniform dye-dependent characteristics and are incompatible with Triton X-100 permeabilization, and 3) WGA Alexa-conjugated solutions which require uniformity of cell surface glycan-expression in order to produce consistent results. MemGlow™ labeling is extremely efficient and even nanomolar concentrations enable visualization of intercellular filopodia and nanotubes (Figure 2).
Figure 2. 3D reconstruction of cells stained with MemGlow 488™ and imaged with laser scanning confocal microscopy. Live KB cells stained with 50 nM MemGlow™ 488 for 5 minutes and imaged without washing. Intercellular filopodia and nanotubes are visible between cells (white arrows). Image false-colored to orange. Image provided courtesy of M. Collot, CNRS, France.
| Probe | Supplier | MemGlow™ advantages |
MemGlow™ | Cytoskeleton Inc. |
|
DiI, DiO & analogs (long-chain carbocyanines) | Thermo Fisher |
|
| WGA Alexa Fluor | Thermo Fisher |
|
| PKH | Millipore Sigma |
|
Table 1. Advantages of MemGlow™ probes compared to popular membrane staining probes.
Flipper TR™
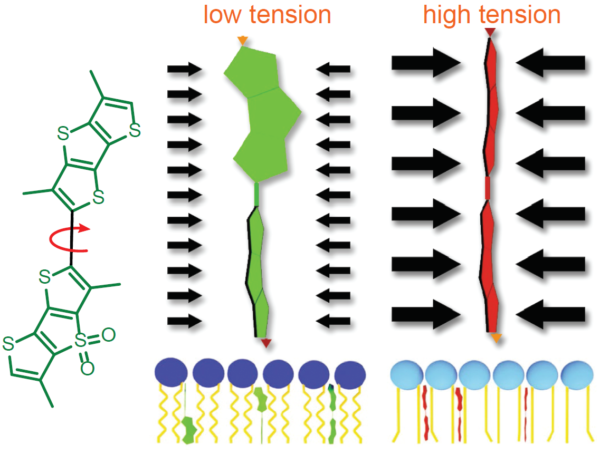
Measurement of membrane tension is historically difficult to perform and requires specialized training. Fortunately, a modern fluorescent probe improves accessibility to membrane tension measurements. The Fluorescent LIPid Tension Reporter, or Flipper TR™, is a live cell fluorogenic membrane tension probe which breaks down technical barriers by technical feats achieved by highly trained biophysicists1. Flipper TR™’s chemical structure permits probes to exhibit two distinct fluorescence lifetimes depending upon their structural status (Figure 3). Flipper TR™ probes integrated within a low-tension cell membrane assume semi-planarized states exhibiting relatively short fluorescence lifetimes. Conversely, when membrane tensions are high, membrane phospholipid pressures force Flipper TR™ probes convert into planarized conformations, resulting in substantial increases in fluorescence lifetimes that can be quantitated and visualized using a FLIM microscope. MDCK cells probed with Flipper TR™ and exposed to increased tension via osmotic shock 0 to 0.6 mN·m-1 exhibited a lifetime fluorescence slope delta of 2.38 ± 0.18 ns·m·mN–1 15. In addition to reporting membrane tension, Flipper TR™ is poised to help investigators unravel the mysteries of lipid rafts as the probe’s utility extends to reporting the membrane lipid phases, lipid ordered (Lo) or lipid disordered (Ld).
NR Probes
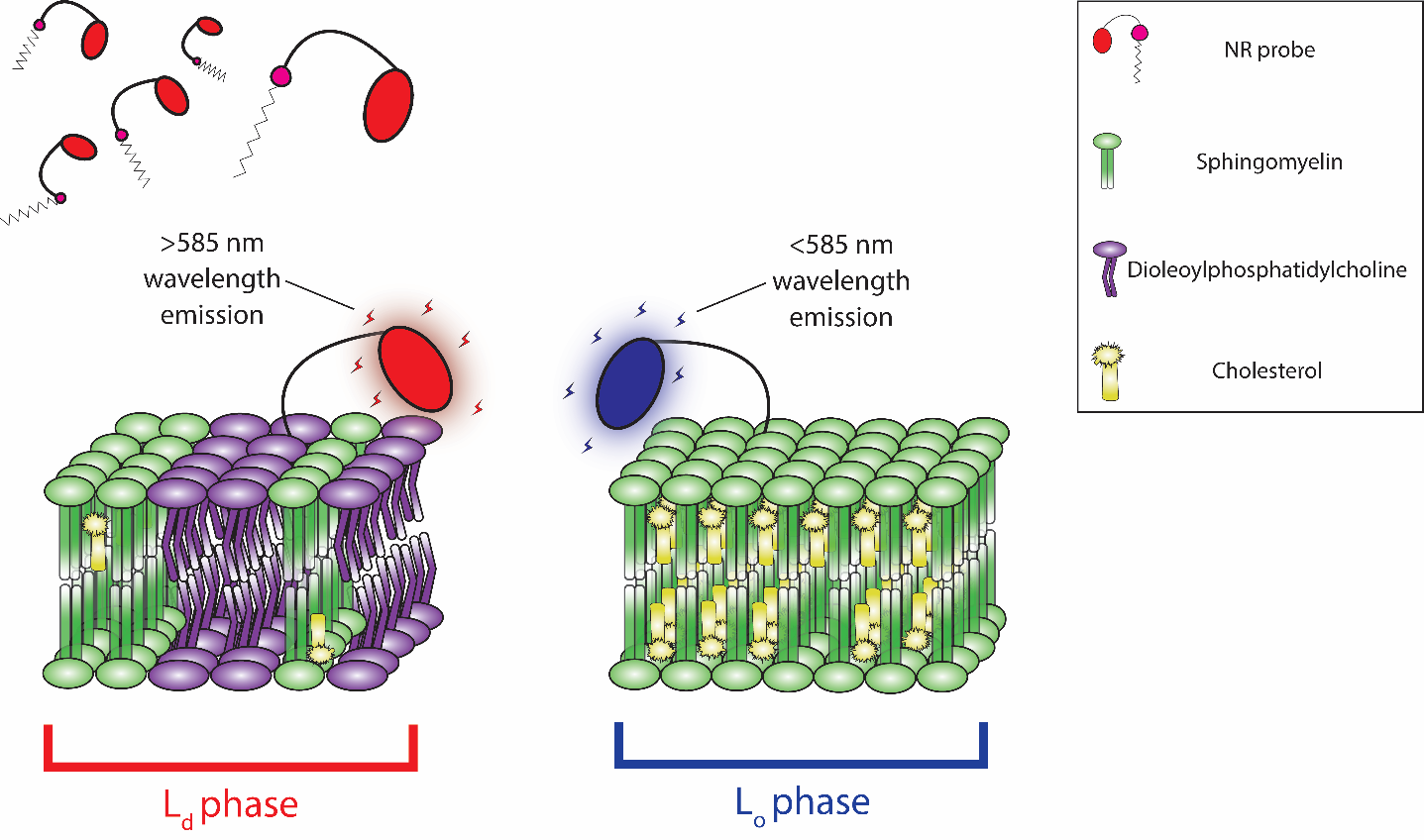
The NR family of probes were developed by structurally enhancing the Nile Red dye. Similar to Nile Red, NR probes readily intercalate into the plasma membrane and undergo a solvachromatic shift in emission wavelength, but their distinct chemical structures provide enhanced capabilities (Figure 4). NR12A and NR4A utilize a new membrane targeting moiety consisting of a sulfonate group, a long (NR12A) or short (NR4A) lipophilic alkyl chain, and lack the destabilizing phenolic oxygen group found in their progenitor molecule. Although structurally similar, NR4A’s shorter alkyl chain provides an easily reversible plasma membrane binding that reflects its purpose-built application for single-molecule localization microscopy (SMLM) and nanoscale cartography of plasma membrane lipid order through spectrally resolved points accumulation for imaging in nanoscale topography (SR-PAINT). During SR-PAINT, the shorter alkyl chain and reversible binding of NR4A enables continual replenishment of depleted probes with unbleached replacement probes from surrounding cell media. Intriguingly, NR4A has permitted investigators to resolve dynamic nano-scale endocytic events in live cells5. To contrast, the longer alkyl lipophilic chain found in NR12A and NR12S confers quasi-irreversible binding that is more suited to conventional microscopy. NR12S has undergone extensive validation revealing a strong bias towards the outer leaflet in lipid bilayers, with low endocytic internalization, and exhibits only marginal flip-flop over hours6. NR12S and its newer NR12A analog enables investigators to gain real-time insights into lipid order without cumbersome, invasive, and inaccurate plasma membrane fractionation assays. Altogether, the new fluorescent NR probes will provide: 1) high plasma membrane specificity in live cell imaging, 2) distinguish between Lo and Ld phases through solvatochromic blue-shifting, and 3) exhibit good photostability and brightness with low background in their respective applications.
Summary
The evolution of membrane probes has occurred over the past 40 years from WGA derivatives, to lipid analogs, and finally to fluorogenic lipid and membrane physical sensors. The most advanced probes now have low background, low toxicity, and enable biophysical and high-resolution microscopy techniques to be used.
References
- Honig, M. G. & Hume, R. I. Dil and DiO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 12, 333–341 (1989).
- Honig, M. G. & Hume, R. I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J. Cell Biol. 103, 171–187 (1986).
- Okun, L. M. Identification and isolation in vitro of neurons marked in situ by retrograde transport. New Approaches Dev. Neurobiol. 109–121 (1981).
- Collot, M. et al. MemBright: A Family of Fluorescent Membrane Probes for Advanced Cellular Imaging and Neuroscience. Cell Chem. Biol. 26, 600-614.e7 (2019).
- a) Colom A et al. 2018. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125. 5b) Danylchuk, D. I., Moon, S., Xu, K. & Klymchenko, A. S. Switchable Solvatochromic Probes for Live-Cell Super-resolution Imaging of Plasma Membrane Organization. Angew. Chemie - Int. Ed. 58, 14920–14924 (2019).
- Kucherak, O. A. et al. Switchable nile red-based probe for cholesterol and lipid order at the outer leaflet of biomembranes. J. Am. Chem. Soc. 132, 4907–4916 (2010).
- Bains, G., Lee, R. T., Lee, Y. C. & Freire, E. Microcalorimetric study of wheat germ agglutinin binding to N-acetylglucosamine and its oligomers. Biochemistry 31, 12624–12628 (1992).
- Wu, Z. L. et al. Differential Distribution of N- and O-Glycans and Variable Expression of Sialyl-T Antigen on HeLa Cells—Revealed by Direct Fluorescent Glycan Imaging. Glycobiology (2020) doi:10.1093/glycob/cwz110.
- Protocol Guide: Exosome Labeling Using PKH Lipophilic Membrane Dyes | Sigma-Aldrich. https://www.sigmaaldrich.com/technical-documents/protocols/biology/cell-culture/exosome-labeling-pkh.html.
- Sigma-Aldrich. PKH26 Red Fluorescent Cell Linker Kits for General Cell Membrane Labeling Catalog. in Technical Bulletin 1–4.
- Dehghani, M., Gulvin, S. M., Flax, J. & Thomas, R. Exosome labeling by lipophilic dye PKH26 results in significant increase in vesicle size. BioRxiv (2019) doi:10.1101/532028.
- Collot, M. et al. Bright fluorogenic squaraines with tuned cell entry for selective imaging of plasma membrane vs. endoplasmic reticulum. Chem. Commun. 51, 17136–17139 (2015).
- Thermo Fisher. D282 Documents & Support and Product FAQs | Thermo Fisher Scientific. https://www.thermofisher.com/search/results?query=D282&persona=DocSupport&type=Product+FAQs.
- D275 Documents & Support and Product FAQs | Thermo Fisher Scientific. https://www.thermofisher.com/search/results?query=D275&persona=DocSupport&navId=4294959582&focusarea=Product FAQs.
- Colom, A. et al. A fluorescent membrane tension probe. Nat. Chem. 10, 1118–1125 (2018).