Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy
- By Cytoskeleton Inc. - Signal-Seeker News
- Oct 22, 2020
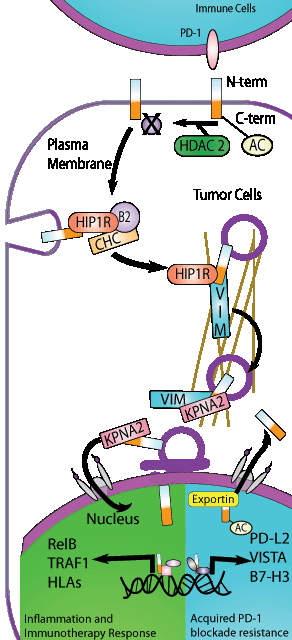
Programmed death-ligand 1 (PD-L1) is a transmembrane protein that binds to the inhibitory checkpoint receptor PD-1; and has been shown to be utilized by cancer cells to avoid immune surveillance and promote tumorigenesis. Checkpoint inhibitor drugs that prevent PD-1/PD-L1 interaction has shown tremendous therapeutic benefit, but primary resistance and secondary relapse to these types of treatments suggest there is still more to learn about these proteins. Recently, Gao et al. reported that PD-L1 can translocate to the nucleus and actively regulate transcription of an array of immune and inflammatory genes to further modulate immune response; importantly, the primary regulatory mechanism of nuclear-PD-L1 translocation was a single acetylation of its c-terminal tail at Lysine 263. An array of genetic, molecular, and pharmacologic tools was utilized to define the acetyl transferase, p300, and the deacetylase, HDAC2, that modifies PD-L1. In-depth studies identified key proteins such as HIP1R, the cytoskeletal protein vimentin, and importin-α1 which all played an active role in transporting deacetylated PD-L1 from the cell surface to the nucleus. ChiP-seq was utilized to identify de novo PD-L1 binding motifs, target genes, and partner transcription factors to further define its role in the nucleus. Physiologic relevance was determined through combinatorial drug therapies where HDAC2 inhibitors had a synergistic effect when combined with PD-1 antibody therapies in mouse models. Acetylation of PD-L1 was first reported by Horita et al. and was discovered using Cytoskeleton’s Signal-Seeker Acetylation Detection Kit (Cat. # BK163). Identifying key post-translational modifications of therapeutically and biologically important targets like PD-L1 is critical as it may reveal key regulatory mechanisms that provide a rationale for combinatorial drug therapies, and ultimately improve clinical outcomes.
Link to the Citation:
Products Mentioned:
For more information, please see our previous citation on PD-L1