Altered succinylation of mitochondrial proteins, APP and tau in Alzheimer’s disease
- By Cytoskeleton Inc. - Tubulin News
- Apr 21, 2022

Reduced glucose metabolism in specific brain regions has been shown to correlate with clinical cognitive dysfunction, and preclinical research studies showed that reduced glucose metabolism can amplify learning and memory deficits. As a result, there is increasing interest in defining the precise metabolic pathways involved in the pathogenesis of neurological diseases like Alzheimer’s disease (AD). A recent study by Yang et al. showed that succinylation, a metabolism-associated post-translational protein modification, provides a potential link between abnormal metabolism and AD pathology. Proteomic analysis of brain tissue from AD and control patients showed interesting and defined changes in succinylation profiles in AD patients. Succinylation of multiple proteins in the mitochondria, an organelle rich in succinylated proteins, declined in patients with AD. Surprisingly, in AD patients, succinylation of a small number of cytosolic proteins increased, and two targets with the largest increases were amyloid precursor protein (APP) and microtubule-associated tau. The group identified lysine, K612, that is succinylated on APP and showed that this modification promoted Aβ accumulation and plaque formation through disrupting its normal proteolytic processing. They also identified lysine, K311, residue within a critical hexapeptide region of tau was highly succinylated in nine out of 10 AD patients but not in any of the control matched patients. Utilizing transgenic tauopathy mouse models, they showed succinylation at K311 correlated with other AD markers. Heparin-induced thioflavin S tau aggregation assays were used to shown K311 succinylation promoted tau’s aggregation. Normal tau function was assessed with in vitro tubulin polymerization biochemical studies, and the results showed that succinylated tau is incapable of promoting microtubule assembly. Collectively these findings indicate that the metabolism-linked succinylation PTM is globally changed in AD and has a profound effect on key proteins associated with AD. Cytoskeleton’s purified and active tubulin (Cat. # T240) was an essential tool used to define how succinylation of tau augmented its ability to promote microtubule assembly.
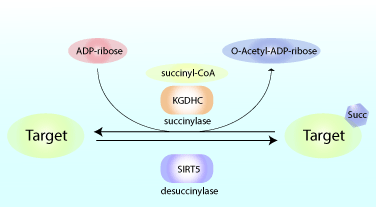
Above: Schematic of Succinylation and Desuccinylation of target proteins
