Citation Spotlight: Arp2/3 regulates RhoA activity and protein levels through cortactin/p190RhoGAP and CCM2/Smurf1, respectively
- By Cytoskeleton Inc. - Small G-Protein News
- Jan 2, 2020

 |
|
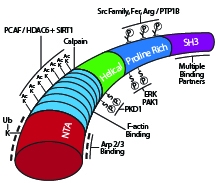
Schematic diagram of cortactin’s primary structure with key post-translational modifications and binding domains highlighted. |
Recently, the potential for the actin cytoskeleton (e.g., actin-binding protein complex Arp2/3) to regulate the activity and protein expression of upstream Rho-family GTPases (e.g., RhoA, Rac1, Cdc42) was evaluated. Genetic or pharmacological inactivation of Arp2/3, which controls actin filament branching, reduced contractility and correlated with decreased myosin II and RhoA (but not Rac1 or Cdc42) activation. The GTPase-activating protein p190RhoGAP reduced RhoA activity through increased physical interactions between the two proteins. P190RhoGAP is normally inhibited via direct cortactin binding, but following Arp2/3 inhibition, cortactin dissociated from p190RhoGAP. Surprisingly, RhoA (but not Rac1, Cdc42, or p190RhoGAP) protein levels increased due to reduced RhoA ubiquitination mediated by the adaptor protein CCM2 (cerebral cavernous malformation 2) and the E3 ubiquitin ligase Smurf1 and subsequent proteasomal degradation. Furthermore, Arp2/3 inhibition and concomitant reduction in active RhoA/increase in total RhoA resulted in defects in cytokinesis which were rescued with a cell-permeable Rho activator. Cytoskeleton’s RhoA, Rac1, and Cdc42 antibodies; rhotekin-RBD and PAK-PBD beads; Acti-stain phalloidins; and Rho activator II (Cat.# ARH04, ARC03, ACD03, RT02, PAK02, PHDG1, PHDR1, and CN03, respectively) were essential in identifying a specific feedback loop between Arp2/3 and RhoA that reveals a previously unknown actin-based regulatory role in RhoA GTPase-mediated signal transduction.
Products used in this citation:
Anti-Cdc42: mouse Mab - (Cat. # ACD03)
Anti-RhoA: mouse Mab - (Cat. # ARH04) - Replaced by ARH05 - available here
