Endothelial Notch signaling directly regulates the small GTPase RND1 to facilitate Notch suppression of endothelial migration
- By Cytoskeleton Inc. - Small G-Protein News
- Jan 26, 2023

The process of new blood vessel growth from the existing vasculature known as angiogenesis occurs in both health and disease as a mechanism to supply surrounding tissue with the necessary exchange of nutrients and metabolites. One of the key components in sprouting angiogenesis is the proliferation, migration, and signaling changes that occur in endothelial cells. The Notch signaling pathway has been identified as a key regulator to control angiogenesis, but the mechanisms are complex and not fully defined. Recent work by Swaminathan et al. investigated how dynamic Notch signaling activates critical signaling mechanisms to suppress endothelial function. Specifically, transcriptional changes were analyzed in control versus Notch-activated HUVEC cells, and many established and novel targets were identified. Additional RNA-seq in vivo studies were also performed and the compilation of the various experiments identified 12 genes that were significantly regulated by Notch. Of the 12 genes, the small GTPase RND1 had the largest response to Notch activation. Further analysis identified a Notch 1 binding site in the enhancer of RND1, and supported chromatin immunoprecipitation studies that showed Notch regulates RND1 levels through direct transcriptional regulation. Knockdown of RND1 was utilized to show that it plays a key role in Notch’s ability to suppress Ras signaling. Interestingly, RND1 had no effect on Notch-regulated proliferation but did affect Notch’s ability to suppress sprout number and length, and migration. Collectively, these data identified RND1 as a critical target of Notch; whereby, it plays a significant role in Notch’s ability to control sprouting angiogenesis. Cytoskeleton Inc’s Ras G-LISA Activation Assay Kit (Cat. #BK131) was essential to measure how RND1 affected Notch’s ability to suppress RAS activation.
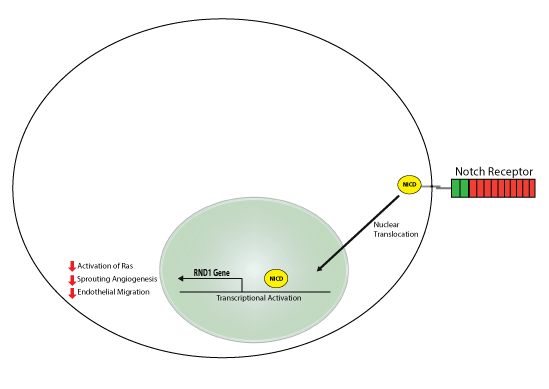
Above: This study identified the small GTPase RND1 as a direct transcriptional target of Notch signaling, furthermore, RND1 mediates Notch-induced RAS signaling, sprouting angiogenesis, and endothelial migration during dynamic Notch activation.
Link to Citation:
Related Products/Products Used
Ras G-LISA Activation Assay Kit (Colorimetric Based) (Cat. # BK131)
RhoA G-LISA Activation Assay Kit (Colorimetric Format) (Cat. # BK124)
