Invasion by actin-driven membrane protrusions: Cortactin in focus
- By Cytoskeleton Inc. - Actin News
- Aug 30, 2013

Cortactin’s multiple signaling domains The actin binding protein cortactin plays an important role in several cellular functions involving plasma membrane changes that are dependent on a dendritic (i.e., branched) actin network: cell motility employing lamellipodia, clathrin dependent and independent endocytosis, host-pathogen interactions, maintenance of endothelial barrier integrity, and invadopodia-mediated cell invasion1. Cortactin is a monomeric ~80 kDa protein that derives its name from its intracellular colocalization with cortical actin at the periphery of the cell2. The amino terminus of cortactin harbors a domain rich in acidic amino acids (N-terminal acidic domain; NTA) that interacts with the seven subunit Arp2/3 complex that promotes dynamic actin filament branching3. Cortactin’s NTA domain contains a DDW (Asp-Asp-Trp) motif that has been observed in the Arp2/3 binding regions of other known actin nucleation promoting factors (NPFs) as either DDW or DEW (e.g., WASP, N-WASP, Myo3, ActA)4 (Fig. 1). NPFs are divided into two classes based on their role in promoting Arp2/3-mediated filamentous actin (F-actin) branching5. Class I NPFs like those mentioned above have a primary role in promoting Arp2/3 activation to drive filament branching, whereas Class II NPFs like cortactin function in branch formation and stabilization of the dynamic branched actin assembly6,7. Notably, while cortactin can directly activate Arp2/3 mediated F-actin branching, this activity is much weaker than is observed for Class I NPFs6. This is due in part to cortactin lacking the monomeric globular actin (G-actin) binding domain of class I NPFs5. Consistent with cortactin’s role in stabilizing branched filaments, it has a central domain with 6.5 repeats of a 37-residue long sequence that binds F-actin2,4 (Fig. 1). This domain is followed by a helical region and a proline-rich region, containing multiple sites of post-translational modifications (PTMs), and an SH3 domain that is used to recruit other proteins, including the Class I NPF N-WASP to the Arp2/3 complex at the filament branch point (for a list of binding partners, see ref. 8).
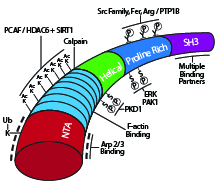
Schematic diagram of cortactin’s primary structure with key post-translational modifications and binding domains highlighted.
