Lysine Acetyltransferase p300 and Its Role in Skeletal Muscle Biology
- By Cytoskeleton Inc. - Signal-Seeker News
- Mar 24, 2016

Recently, LaBarge et al. examined the role of the acetyltransferase E1a-binding protein (p300) in skeletal muscle function and metabolism. Reversible acetylation, a well-known post-translational modification, is considered a regulator of mitochondrial metabolism and exercise-induced adaptation in skeletal muscles. This conclusion is based primarily on data derived from studies of deacetylases and skeletal muscle physiology with a dearth of information on how lysine acetyltransferases impact the same muscle physiology. While whole-body heterozygous and homozygous p300 knockout mice have muscle defects (along with neural and cardiac) and die in embryogenesis, to date, the role of p300 in skeletal muscle function has not been studied with a muscle-specific knock-out mouse model. Here, the authors created such an in vivo model and found that knocking out p300 affected neither the development nor function of adult skeletal muscle. Moreover, it was also not required for mitochondrial adaptation induced by endurance exercising. Cytoskeleton’s anti-acetyl lysine antibody (Cat. # AAC01) was an essential reagent in this study as it was used to confirm a functional loss of p300 in the knock-out mice. These mice had a significant reduction in total acetylation levels in skeletal muscle immunoprecipitates from knock-out, compared to wild-type, mice.
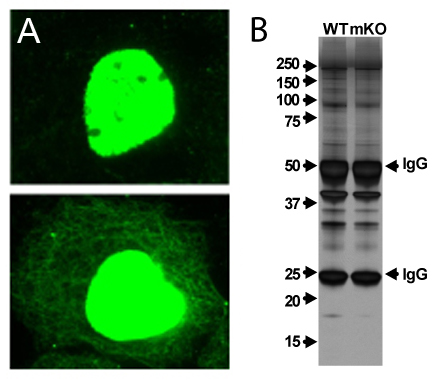
A) Human epidermoid carcinoma A431 cells, untreated (top) or treated (bottom) with 5 M TSA (16 h). Acetylated cytoplasmic and nuclear proteins were visualized in green fluorescence. B) Silver stain of acetyl-lysine immunoprecipitates from wild-type (WT) and muscle-specific knock-out of E1a-binding protein (mKO) mice.
