MICAL1 activation by PAK1 mediates actin filament disassembly
- By Cytoskeleton Inc. - Actin News
- Apr 20, 2023

RHO GTPase family regulation of actin has been studied for many years and has been shown to be a critical regulator of actin. Recent data by Olson and colleagues identify a regulatory link between PAK1 kinase, a downstream effector of Cdc42, and the actin-oxidizing protein MICAL1. The group performed preliminary pull-down studies with a panel of RHO GTPases and downstream effectors in combination with MICAL1. MICAL1 was shown to directly interact with activated PAK1 but not the other proteins, which was confirmed via chemical inhibitors and mutagenesis experiments. Additional mutagenesis experiments were performed to identify critical domains of MICAL1 that were necessary for PAK1 binding, and both the monooxygenase and calponin homology domains were deemed essential. Both sedimentation and actin depolymerization assays were utilized to determine if PAK1 affected MICAL1’s ability to depolymerize actin. In the presence of activated PAK1, MICAL1’s activity was significantly enhanced resulting in robust depolymerization of actin. It was determined that PAK1 regulated MICAL1 activity through phosphorylating MICAL1 at two specific serine sites. This phosphorylation was activated by growth factors such as FGF2 and led to confirmational changes of MICAL1 that allowed for enhanced interaction with numerous proteins including specific RAB family proteins. Collectively, these studies identify a new mechanism by which RHO GTPase family members can target actin regulatory proteins to fine-tune actin dynamics. Cytoskeleton Inc’s pyrene-labeled actin protein (Cat. # AP05) was essential for the in vitro depolymerization studies used to investigate how PAK1 affected MICAL1 function.
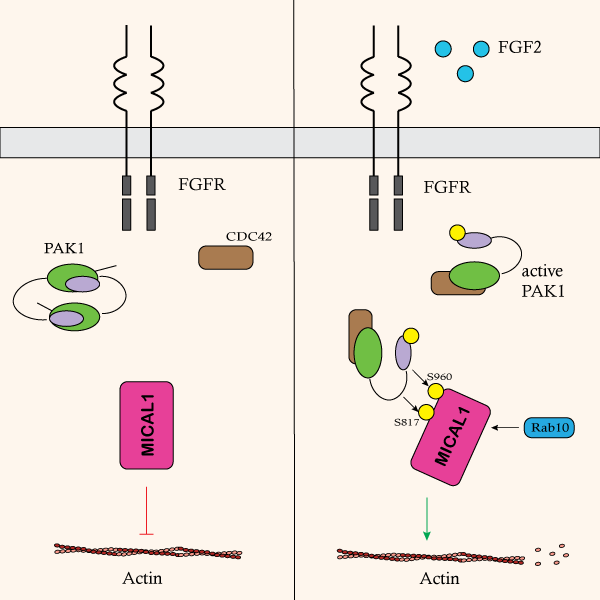
Above: Schematic showing the mechanism by which extacellular signaling pathways activate PAK1 via Cdc42. Active PAK1 binds and phosphorylates MICAL1 resulting in enhanced RAB family binding as well as F-actin disassembly.
Link to Citation:
Products Used in Citation:
Actin Protein (Pyrene Labeled): Rabbit Skeletal Muscle (Cat # AP05)
Actin Protein (>95% Pure): Rabbit Skeletal Muscle (Cat # ALK95)
