O2-Dependent Protein Internalization Underlies Astrocytic Sensing of Acute Hypoxia by Restricting Multimodal TRPA1 Channel Responses
- By Cytoskeleton Inc. - Signal-Seeker News
- Jul 8, 2021

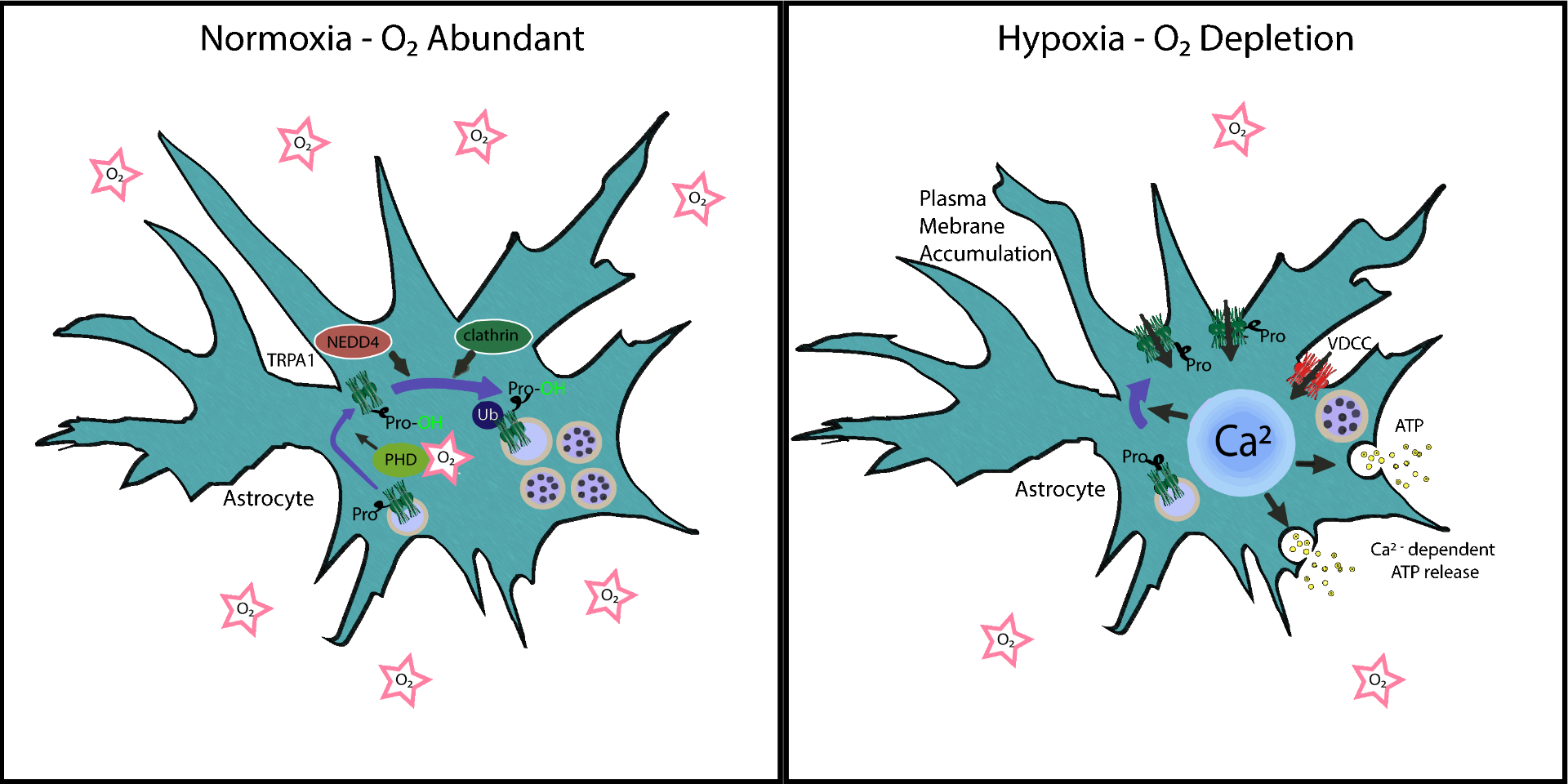
Astrocytic Sensing of Hypoxia by TRPA1 Channels
Oxygen, which is critical for ATP generation in aerobic organisms, is essential for life; conversely deprivation of oxygen, hypoxia, results in impaired cell signaling, energy crisis, and ultimately demise. Specific organs of the body have much higher consumption of O2 such as the central nervous system (CNS) which has high energy demands. Recently, Uchiyama et al. investigated the mechanisms by which neuronal cells regulate and respond to O2 deprivation and determined that a sensor cation channel transient receptor potential (TRP) A1 in pFRG/RTN astrocytes are critical for responding to hypoxic conditions. Interestingly, they determined that under normoxic conditions TRPA1 undergoes proline hydroxylation and internalization from the plasma membrane. Once internalized it is polyubiquitinated by the E3 ubiquitin ligase NEDD4-1 and targeted for degradation. Conversely, under hypoxic conditions the TRPA1 protein rapidly accumulates at the plasma membrane and is no longer ubiquitinated. This accumulation mediates calcium influx, ATP release from astrocytes, and enhanced respiratory center activity. The group went on to utilize mouse models with astrocyte specific TRPA1 knockdown to determine this mechanism is active and critical for proper CNS response during hypoxia. Cytoskeleton’s Signal-Seeker ubiquitination detection kit was critical for measuring TRPA1 ubiquitination under normoxic and hypoxic conditions and helped uncover how the regulation of TRPA1 expression is critical for the hypoxia sensing function of pFRG/RTN astrocytes.
Link to Citation:
Related Products / Products Used:
Signal-Seeker™ Ubiquitination Detection Kit (30 Assay)(Cat. # BK161)