Citation Spotlight: Targeting The Cytoskeleton to Direct Pancreatic Differentiation of Human Pluripotent Stem Cells
- By Cytoskeleton Inc. - Actin News
- Sep 3, 2020

Recently, Hogrebe et al. established a link between the state of the actin cytoskeleton in human pluripotent stem cells (hPSCs) and the expression of pancreatic transcription factors that control pancreatic lineage specification. Effectively differentiating hPSCs into pancreatic beta cells could bring forth the potential of cell therapy for the treatment of diabetes. However, differentiation of hPSCs into stem cell-derived beta (SC-ß) cells is a delicate and precise process that had only been successfully achieved in suspension cell cultures, suggesting that the insoluble microenvironment (found in two dimensional cultures) suppress SC-ß cell fate. Various extracellular matrix proteins, integrin compositions, and manipulation of substrate stiffness were examined, and determined cell attachment to be a critical regulator. A panel of compounds that affect cytoskeletal dynamics were then used to assess the role of the cytoskeleton in progenitor differentiation as determined by measuring levels of the pancreatic transcription factor, NEUROG3. Latrunculin A, a G-actin binding drug, had a profound effect on NEUROG3 levels, identifying actin polymerization as a regulator of hPSCs differentiation. G/F actin ratios were analyzed in several conditions and confirmed that the G/F-actin ratio correlated with NEUROG3 expression. Interestingly, they found that the actin polymerization states at specific stages of differentiation was critical for proper differentiation to SC-ß cells and improper actin polymerization at these stages significantly diminish the percentage of cells that become insulin producing SC-ß cells. This work ultimately led to the development of the first successful two-dimensional differentiation protocol for generating SC-ß cells that were able to reverse severe pre-existing diabetes in mice similarly to human islets and roughly three times faster than SC-ßs generated with the conventional suspension protocol. Cytoskeleton's G-Actin/F-Actin In Vivo Assay Biochem Kit (Cat. # BK037) was an essential reagent in this study, providing critical evidence of a correlation between G/F-actin levels and NEUROG3 expression.
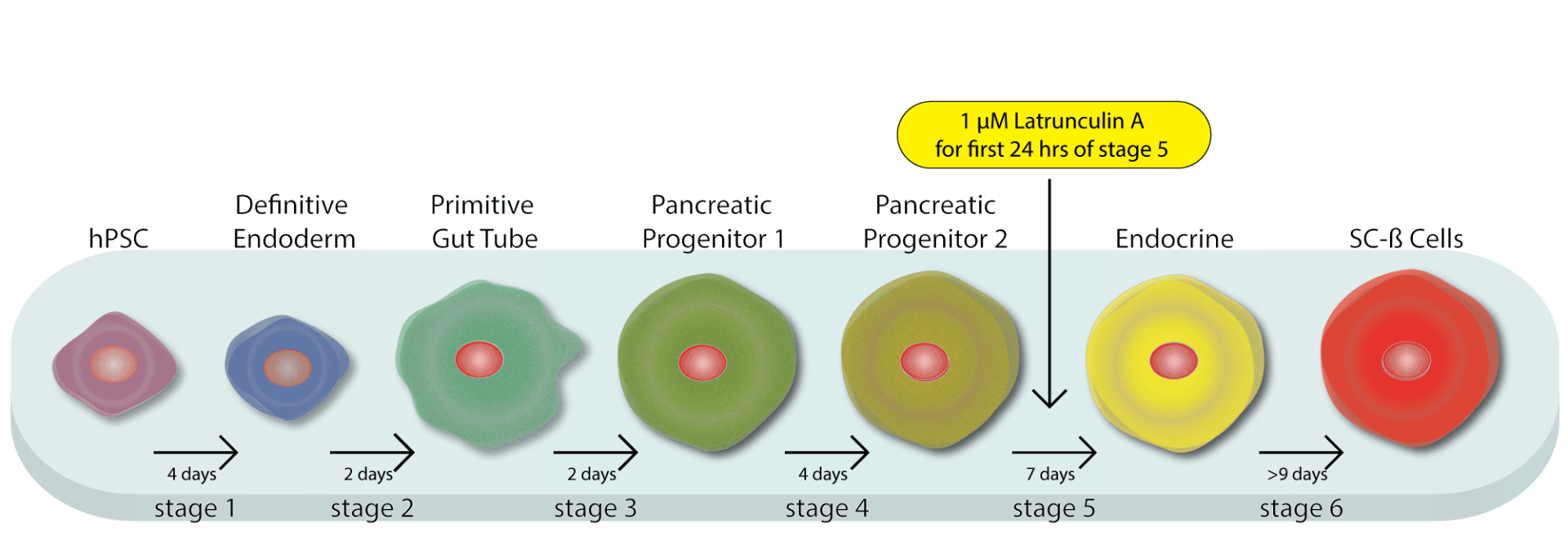
Schematic of new planar protocol for making SC-ß cells
