Citation Spotlight: PD-L1: Regulation by the Post-Translational Modification of Monoubiquitination
- By Cytoskeleton Inc. - Signal-Seeker News
- Mar 29, 2017

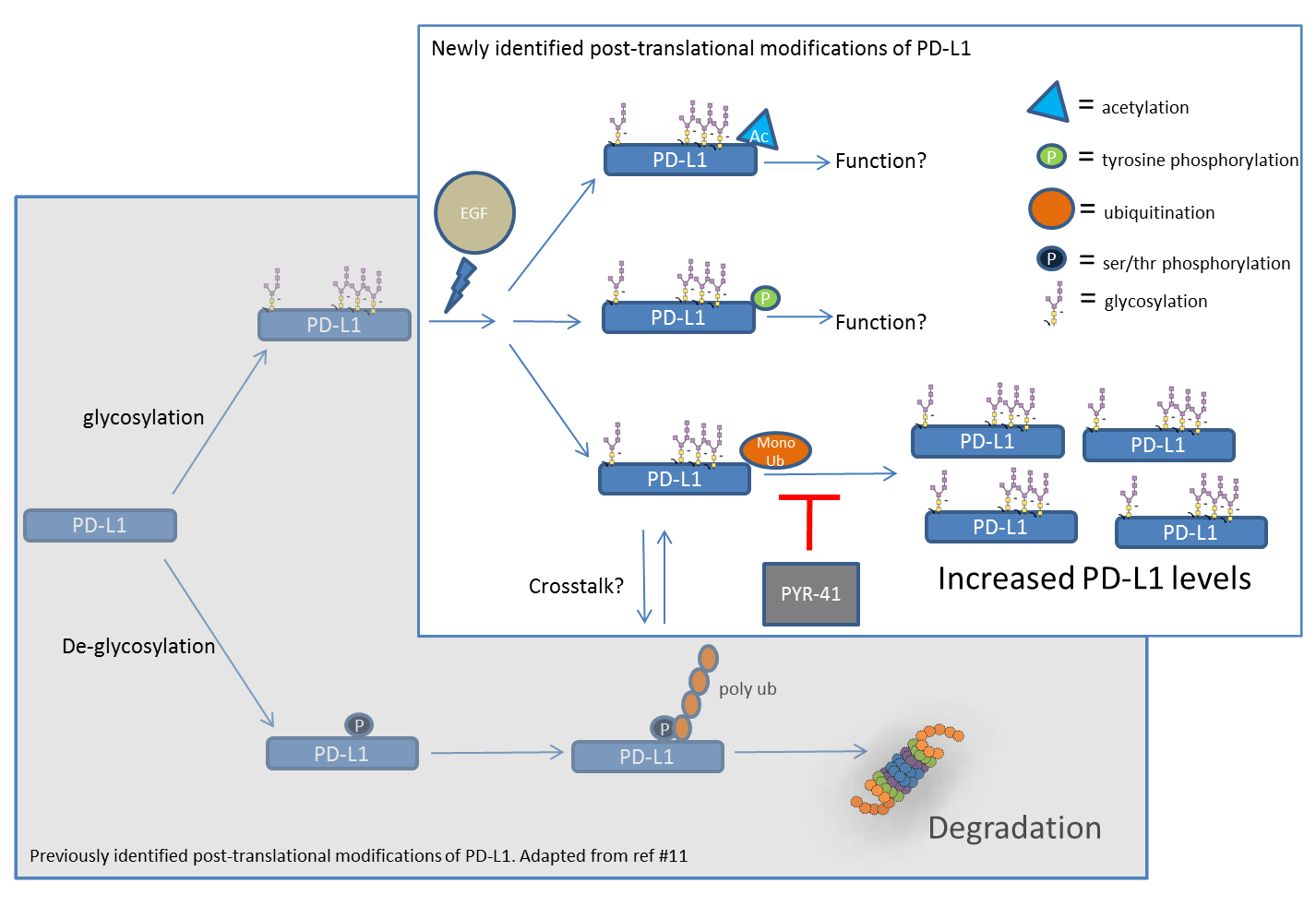
Model: Profile of PD-L1 post-translational modifications and their roles in regulating PD-L1 protein levels.
Recently, Horita et al. profiled four post-translational modifications (PTMs) of the programmed cell death ligand 1 (PD-L1) protein, an important immune checkpoint inhibitor and key target in anti-cancer treatments. Using a set of high-affinity, high-specificity endogenous PTM detection reagents, the authors examined PD-L1's levels of tyrosine phosphorylation, ubiquitination, acetylation, and SUMOylation in A431 cells treated with epidermal growth factor (EGF). These studies led to the novel identification of PD-L1 modified tyrosine phosphorylation, acetylation, and mono- and multi-ubiquitination. Critical temporal studies led to the observation that mono- and multi-ubiquitination preceded an EGF-stimulated increase in total PD-L1 protein expression. Pharmacological inhibition of the EGF receptor (EGFR) activation further demonstrated that mono- and multi-ubiquitination of PD-L1 relies upon EGFR activation. Importantly, inhibition of ubiquitin E1 activating enzyme blocked any increase in total PD-L1 protein, revealing a potential mechanistic role for ubiquitinated PD-L1 in the regulation of total PD-L1 protein levels. Cytoskeleton's Signal Seeker kits (Cat.# BK160, BK161, BK162), EGF (Cat.# CN02), anti-acetyl lysine antibody (Cat.# AAC01), and anti-tubulin antibody (Cat.# ATN02) were essential reagents in this study, providing the tools necessary for an insightful, novel characterization of PTMs that can regulate PD-L1, an important protein in immune homeostasis, and anti-cancer therapies.
