RhoA and Cdc42 Activity Mediates Podoplanin-induced Tumor Progression
- By Cytoskeleton Inc. - Small G-Protein News
- Feb 2, 2016

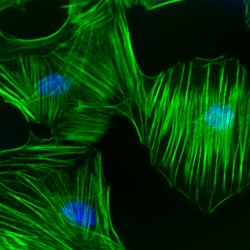
Recently, Li et al. examined the signaling pathways underlying podoplanin-mediated tumor invasion in oral squamous cell carcinoma (OSCC) tissues and cell lines. Overexpression of podoplanin, a transmembrane glycoprotein, characterizes multiple cancers; however, its exact role(s) in tumor progression/invasion remain unknown. Here, podoplanin overexpression increased tumor cell protrusions (i.e., invadopodia) and F-actin stress fibers in OSCC cells. Concomitantly, RhoA activity decreased whereas Cdc42 activity increased. Correspondingly, podoplanin knockdown reversed these activity patterns. Rac1 activity did not change after any treatments. Additionally, RhoA and Cdc42 engaged in cross-talk (e.g., RhoA inhibition resulted in increased Cdc42 activity). Activated Cdc42 (but not RhoA) was also found to co-precipitate with matrix metalloproteinase-14 (i.e., MT1-MMP) and binding increased with podoplanin overexpression and decreased with knockdown. Cytoskeleton’s Acti-stain 488 phalloidin (Cat.# PHDG1) and RhoA/Rac1/Cdc42 Activation Assay Kit (Cat.# BK030) were essential reagents in this study, providing: 1. detection of morphological changes in the actin cytoskeleton necessary for increased cellular protrusions and stress fibers; and 2. confirmation that this re-organization corresponded with opposing changes in RhoA and Cdc42 activity. Thus, podoplanin-mediated tumor cell protrusion and resulting motility relies upon an activation and inhibition of Cdc42 and RhoA, respectively, which, in turn, mediate the re-organization of the actin cytoskeleton.