RHOA-mediated mechanical force generation through Dectin-1
- By Cytoskeleton Inc. - Small G-Protein News
- Jan 26, 2021

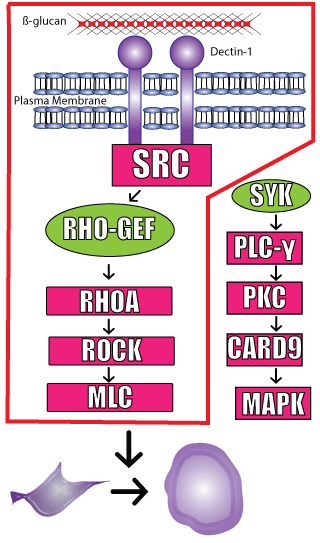
Pattern Recognition Receptors (PRRs) are important protein components of the innate immune system that are capable of recognizing molecules frequently found in pathogens. PRRs, commonly expressed on immune cells, become activated by binding to specific pathogen molecules which leads to a variety of responses in the cell such as phagocytosis, oxidative burst and production of inflammatory cytokines. One such PRR molecule, Dectin-1, specifically recognizes b-glucan, a highly abundant protein found in the cell walls of Candida albicans, a pathogenic yeast that produces high rates of fungal infections in people. Recently, Choraghe et al. investigated the molecular signaling pathways utilized by Dectin-1 to promote fungus internalization in response to b-glucan stimulation. Their initial studies utilized a heterologous cell line, HEK293, with or without Dectin-1 expression to deduce that b-glucan induced morphological changes were dependent on the PRR. They then used a panel of chemical inhibitors to identify which of the established downstream signaling pathways activated the contractile mechanical forces that promoted cellular contraction. Interestingly, the conical SYK dependent kinase was dispensable; conversely, SFK downstream signaling appeared to be important for the morphological changes. To further define the regulatory pathway they looked at two force generating mechanisms; the Ca2+/calmodulin and RHOA/ROCK pathways. Ca2+/calmodulin inhibition did not alter b-glucan stimulated contraction; however, inhibition of the RHOA/ROCK pathway with a C3 transferase, RHOA specific inhibitor (Cat # CT04) abolished all morphological changes. This phenomenon was replicated utilizing ROCK inhibitors, and these studies were further validated in M1 macrophages as well. The group then utilized RHOA (Cat # BK124), CDC42 (Cat # BK127), and Rac1 G-LISA (Cat # BK126) activation assays to verify that b-glucan stimulation led to RHOA activation, but not CDC42 or Rac1. Lastly, the group used phagocytosis assays to show RHOA inhibition was sufficient to completely abolished phagocytotic cupping in the presence of b -glucan; thus, further implicating RHOA-ROCK-MLC as a critical pathway utilized by Dectin-1 to initiate cellular events. The panel of G-LISA toolkits and C3 transferase specific RHOA inhibitor from Cytoskeleton was essential in determining the specific signaling mechanism utilized by the innate immune system PRR to initiate critical cellular morphological changes in response to pathogenic insults.