ROCK1 mechano-signaling dependency of human malignancies driven by TEAD/YAP activation
- By Cytoskeleton Inc. - Actin News
- Jul 19, 2022

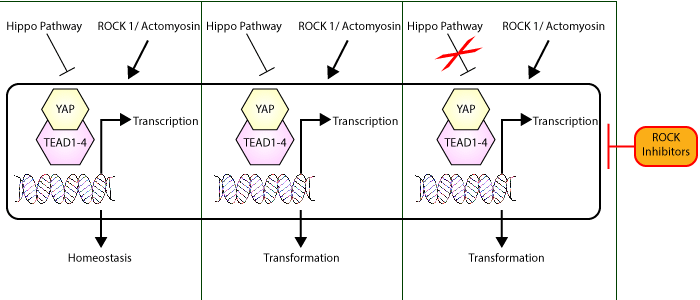
Targeting specific signaling pathways to treat cancer has led to an array of new therapeutics with promising outcomes. Recently, Esposito et al. sought to investigate the mechanism by which a group of P53 mutants activated the TEAD/YAP transcriptional machinery, and discovered that they do so indirectly through ROCK1-dependent mechanisms as a means to drive oncogenesis. These p53 missense mutations impair p53’s ability to bind its target promoter regions and in their screen were found to enhance TEAD transcription that was mediated via the mevalonate (MVA) pathway. They then utilized inhibitors such as simvastatin and GGTI-298 to inhibit MVA and prenylation respectively to disrupt TEAD/YAP transcriptional activation in cells expressing these p53 mutants, which provided additional evidence of the importance of MVA and led to the detection of RhoA dependent signaling as a critical pathway. RhoA’s importance was confirmed through actin polymerization studies. This pointed them towards investigating ROCK1 signaling, which they found was active in their model. Additionally, in tumors where hippo pathway signaling is dysfunctional, they also observed elevated TEAD/YAP activation that was dependent on RhoA/ROCK1/actomyosin signaling. ROCK1 inhibitors were sufficient to reduce TEAD/YAP activity in both their p53 and hippo signaling models, suggesting a critical role for the RhoA signaling pathway in TEAD/YAP-dependent tumors. Cytoskeleton’s G-Actin/F-Actin In Vivo Assay Biochem Kit (Cat. #BK037) was essential to characterize actin polymerization levels in their models to validate functional RhoA signaling. As investigators continue to pursue the targeting of critical oncogenic drivers to combat cancers, studies like this that identify critical oncogenic drivers and potential therapeutic targets provide invaluable information.