Citation Spotlight: Vav2 GEF Binds Phosphorylated Cortactin for Activation of Rac3 in Invadopodia
- By Cytoskeleton Inc. - Small G-Protein News
- Nov 21, 2017

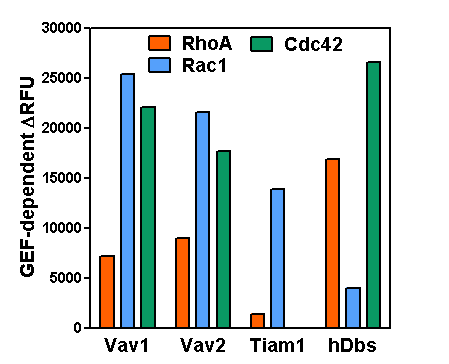
Rosenberg et al. recently investigated the molecular mechanism responsible for cortactin’s regulation of actin polymerization underlying the maturation and function of invadopodia in breast cancer cells. Release of epidermal growth factor (EGF) stimulates formation of invadopodia, actin-enriched cell protrusions essential for extracellular matrix degradation and cancer cell invasion. The EGF receptor-Src-Arg kinase signaling cascade results in phosphorylation of cortactin. The authors found that two tyrosine-phosphorylated residues on cortactin (Y421 and Y466) bind the SH2 domain of Vav2, a guanine nucleotide exchange factor (GEF) for Rho-family GTPases such as Rac. Tyrosine-phosphorylated cortactin recruits Vav2 to invadopodia, which facilitates their maturation and subsequent invadopodia-mediated matrix degradation and cancer cell invasion. Invadopodial function depends upon re-arrangement of the actin cytoskeleton, all of which requires phospho-cortactin-mediated recruitment of Vav2 to the invadopodia. Furthermore, Vav2’s regulation of actin cytoskeletal dynamics involves Rac3 activation, though the exact role of GTP-bound Rac3 remains unclear. Cytoskeleton’s RhoGEF Exchange Assay Kit and biotinylated actin (Cat. # BK100 and AB07, respectively) were essential reagents in this study, providing the tools necessary to identify Vav2’s substrate targets and effects on actin cytoskeletal dynamics to aid in the development of anti-cancer therapeutics that act by interfering with invadopodia function.
Products used in this citation:
RhoGEF Exchange Assay (Cat. # BK100)
Actin Protein (Biotin): Skeletal Muscle (Cat. # AB07)
Products in the GEF Protein Series:
