Citation Spotlight: Wnt5a regulation of RhoA-mediated remodeling of actin cytoskeleton to control ESCC cell motility
- By Cytoskeleton Inc. - Small G-Protein News
- Jan 6, 2020

Recently, Wu et al. investigated if Wnt5a signaling affects migration of esophageal squamous cell carcinoma (ESCC) cells and the molecular pathways underlying any Wnt5a-mediated effects. Wnt5a regulates the motility and invasive behavior of breast cancer and glioblastoma cells. A potential role for Wnt5a in ESCC cell migration remained to be demonstrated. Using a variety of pharmacological, molecular, cellular, and biochemical techniques, the authors establish that Wnt5a positively regulates the in vitro invasive behavior of multiple ESCC cell lines. Specifically, Wnt5a expression is up-regulated in invasive tumor tissues (versus non-invasive tumor tissues), resulting in activation of the Wnt5a receptors, ROR1/2 (receptor tyrosine kinase-like orphan receptor 1/2). These changes are the first steps in the sequential activation of a signaling cascade consisting of DAAM1 (disheveled 2/disheveled-associated activator of morphogenesis 1), the GTPase RhoA (but not Rac1, Rac2, or Cdc42), and finally RhoA-mediated re-organization of the actin cytoskeleton. Importantly, ESCC cell invasion requires the participation of each protein component in the above signaling cascade. Cytoskeleton’s RhoA, Rac1,2,3, and Cdc42 G-LISA activation assay kits (Cat. # BK121, BK125, and BK127, respectively) were essential reagents in this study which identified ROR1/2 as novel targets for therapeutic intervention in multiple cancers.
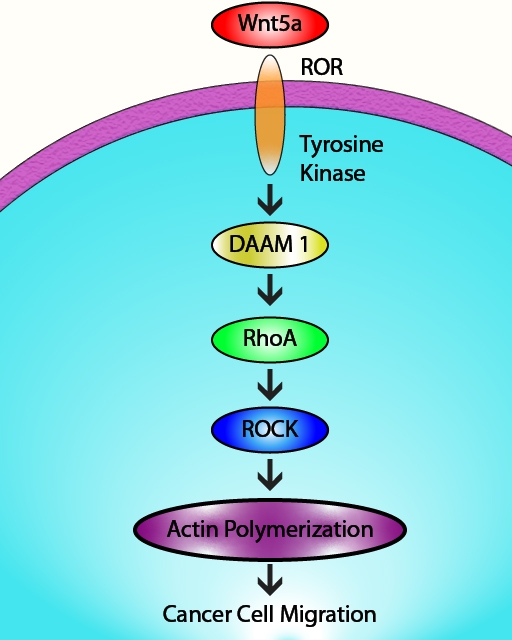
RhoA-mediated actin cytoskeleton remodeling is regulated by a Wnt5a signaling cascade.
