Citation Spotlight: Xenopus Egg Cytoplasm Self-assembles into Spatially Organized, Cell-like Compartments De Novo
- By Cytoskeleton Inc. - Live Cell News
- Jan 2, 2020

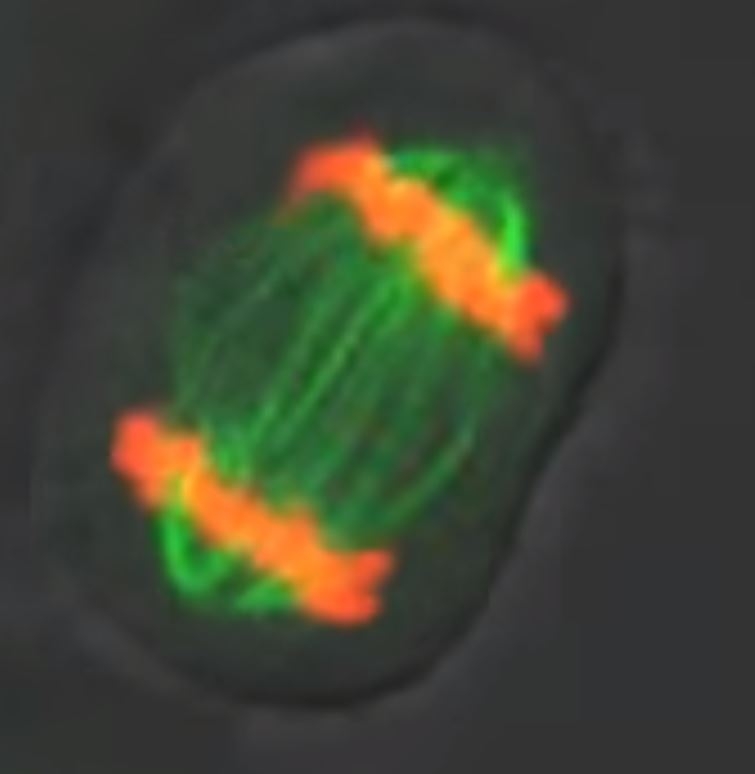
Recently, Xenopus laevis eggs were used to study if homogenized egg cytoplasm can support spontaneous cellular re-assembly with restored macromolecular structures and functionality. Bright-field and fluorescent microscopy demonstrated that the cytoplasm of eggs arrested at interphase and homogenized can undergo self-assembly into sheets of 300-400 micron long, cell-like compartments in ~30 minutes. Successful self-assembly required formation of microtubules (MTs), but not F-actin, minus-end-directed cytoplasmic dynein motor activity, and adenosine triphosphate. MTs in the cell-like compartments appeared similar to the MTs of intact Xenopus embryos. Neither nuclei (chromatin) nor centrosomes were needed for self-assembly initiation. Genomic input was minimized, if not fully precluded, by inhibition of protein translation with cycloheximide. Introduction of demembranated sperm nuclei as a source of centrosomes and DNA enabled the cell-like compartments to undergo multiple cycles of mitosis if cycloheximide was excluded. Cytoskeleton’s SiR-actin and SiR-tubulin live cell imaging probes, along with green and far-red fluorescently-labeled tubulins (Cat.# CY-SC001, CY-SC002, TL488M, TL670M, respectively), revealed the dynamic re-organization, subcellular localization, and functional roles of MTs and F-actin in the formation of the cell-like compartments. The study confirms that cytoplasmic components of Xenopus egg extracts provide the necessary macromolecular complexes, spatial organization, and cell cycle function to initiate self-organization.
Products used in this citation: