Myosin - smooth muscle S1 fragment
* Limited stock available. If stock is not available, Cytoskeleton will produce a new batch upon request. Minimum order will apply. Inquire for more information.
Product Uses
- Measurement of F-actin activated myosin ATPase activity
- Identification/characterization of proteins or small molecules that affect myosin ATPase activity
- Identification/characterization of proteins or small molecules that affect myosin / F- actin interaction
Materials
Smooth muscle myosin protein has been purified from chicken gizzards (1, 2). The full length myosin protein was purified with its essential light chains (ELC) and regulatory light chains (RLC), see Figure 1 and 2. Myosin was then digested with papain to liberate the soluble subfragment-1 (S1) domain, which was isolated by centrifugation (3, 4). The purified myosin S1 fragment has been determined to be biologically active in an F-actin activated ATPase assay (see biological activity assay). Chicken gizzard S1 myosinprotein is supplied as a white lyophilized powder.
Figure 1. Diagrammatic representation of the myosin protein and its subfragments
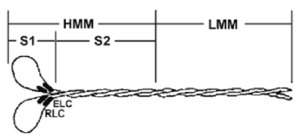
Legend: Myosin is a hexameric protein consisting of two heavy chains and two light chains. Myosin can be proteolytically cleaved into heavy meromyosin (HMM) and light meromyosin (LMM) by α-chymotrypsin in the presence of magnesium. In the presence of EDTA, however, α-chymotrypsin produces the soluble myosin S1 fragment (3).
Storage and Resonstitution
Briefly centrifuge to collect the product at the bottom of the tube. Reconstituting a tube of CS-MYS05 with 180 ul of Milli-Q water will generate a 1.4 mg/ml stock of gizzard S1 myosin in the following buffer: 8 mM PIPES pH 7.0, 8 mM MgCl2, 5% (w/v) sucrose and1% (w/v) dextran. The protein should not be exposed to repeated freeze-thaw cycles. The lyophilized protein is stable at 4°C desiccated(<10% humidity) for 1 year.
Purity
Protein purity is determined by scanning densitometry of Coomassie Blue stained protein on a 4-20% gradient polyacrylamide gel. The myosin and its light chains used to produce the myosin S1 fragment was determined to be 90% pure (see Figure2).
Figure 2. Full length and S1 myosin
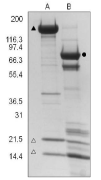
A 20 μg sample of full length Chicken gizzard myosin protein (laneA) and the corresponding S1 myosin (lane B) were separated by electrophoresis using a 4-20% SDS-PAGE gel and stained with Coomassie Blue.The closed triangle indicates the myosin heavy chain (approx. 200kDa), open triangles indicate the light chains (approx. 17 and 20 kDa), and the S1 fragment (approx. 97 kDa) is indicated with a closed circle. Protein quantitation was performed using the Precision Red™ Protein Assay Reagent (Cat.# ADV02). Mark12 molecular weight markers are from Invitrogen.
Biological Activity Assay
The biological activity of chicken gizzard S1 myosin can be determined from its rate of F-actin activated ATP hydrolysis. The assay is constructed by first polymerizing actin to form F-actin. Myosin is then added in substoichiometric amounts and there action is initiated with ATP.
Reagents
1. Gizzard S1 Myosin (0.25 mg, # CS-MYS05)
2. Cardiac Actin (1 mg, # CS-ADMK)
3. ATPase Assay Biochem Kit (Cat. # BK051)
4. 100 mM ATP in 50 mM Tris-HCl pH 7.5
5. PM12 Buffer (12 mM Pipes-KOH, pH 7.0, 2 mM MgCl2).
6. 500 mM EGTA-Na, pH 8.0.
Equipment
1. Spectrophotometer capable of measuring absorbance at 360 nm (+/- 5 nm bandwidth). We recommend a Spectra-Max M2 (Molecular Devices), filter based machines are not suitable.
2. Half area 96 well microtiter plate (Corning Cat.# 3696 or 3697)
3. Multi-channel pipette
Method
The following major steps are recognized:
Step 1. Assemble required chemicals. (30min). Only if screening.
Step 2. Prepare F-actin polymer stock. (2h).
Step 3. Prepare Motor Mix and plate reader. (15min).
Step 4. Pipette Motor Mix into wells and start reaction/platereader. (10min).
F-actin polymer stock
1. Resuspend ADMK or AD99 with 0.5 ml of Buffer to 2 mg/ml, Buffer is 5mM Pipes-KOH, 100 uM ATP, 500uM DTT.
2. Place at RT for 30 min to depolymerize the actin oligos that form during concentration/lyophilization.
3. Then add 2 mM MgCl2 and 2 mM EGTA and incubate at RT for 1 h to polymerize. (shelf life 1h at RT).
Myosin ATPase assay
1. Dilute S1 myosin to 0.5 mg/ml with ice cold PM12 Buffer containing 1mM DTT.
2. Mix the following to make 0.49 ml of actin/myosin mixture:
250 μl of F-actin (2 mg/ml in 5mM Pipes pH 7.5, 2 mM MgCl2, 58 μM CaCl2, 2 mM EGTA, 100 μM ATP, 500 μM DTT). Replaced with equal volume PM12 Buffer for no Factin control.
120 μl of PM12 Buffer
100 μl 5x MSEG
5 μl of 100x PNP
10 ul of S1 myosin (0.5 mg/ml)
3. Add 5 ul of 50 mM ATP and mix.
4. Incubate at 37°C for 3 min.
5. Using the pre-warmed half area 96-well plate, pipette the following:
6. Pipette 10 μl of Milli-Q water into each well.
7. Pipette 90 μl of actin/myosin mixture per well.
8. Start protocol, 41 readings, 30 seconds apart, 37°C , OD 360nm.
9. Calculate Vmax and compare non-activated to F-actin activated samples.
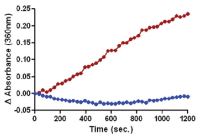
Figure 3 legend: The sarcomere assay was set up as described in the protocol above. Calcium was titrated between 2 and 200 μM and the results plotted on this dose response graph. pCa = 6.1 μM is similar to published pCa values for reconstituted cardiac sarcomeres (Holroyde et al. 1980, Fig.6).
References
1. Persechini, A., and Hartshorne, D.J. 1983. Biochemistry 22: 470-476
2. Trybus, K.M. 1994. J. Biol. Chem. 269:20819-20822.
3. Greene, L.E. et al. 1983. Biochemistry 22, 530-535.
4. Seidel, J.C. 1980. J. Biol. Chem. 255, 4355-4361.
For product Datasheets and MSDSs please click on the PDF links below.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
Coming soon! If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
