Pyrene Muscle Actin (bovine cardiac muscle, >99% pure)
* Limited stock available. If stock is not available, Cytoskeleton will produce a new batch upon request. Minimum order will apply. Inquire for more information.
Product Uses
- Identification and characterization of muscle actin binding proteins
- In vitro actin polymerization studies
- Antibody standard for Western blot analysis
Background Information
Actin is a 43 kDa protein that is very highly conserved between species. There are three main actin isotypes (α, β and γ), which show >90% amino-acid (aa) homology between isotypes and >98% homology within members of a particular isotypic group. The majority of the isotype heterogeneity is located in the amino-terminal 30 residues. The amino-terminus of actin is located on the periphery of the double-helix in F-actin (1) and this site is also thought to interact with myosin (2).
Importantly, different actin isotypes have been shown to behave very differently in vitro and in vivo. Recent studies describe differential subcellular localization of γ-actin (3,4) and isotype specific binding of actin associated proteins. A good example of the latter phenomenon is the report of ezrin specificity for β-actin (5). These studies set a clear precedent that in vitro studies of actin systems should use the actin most closely related to the in vivo isotype.
Globular-actin (G-actin) readily polymerizes under physiological conditions to form Filamentous-actin (F-actin) with the concomitant hydrolysis of ATP. F-actin is a double-helical filament as shown in Figure 1.
Figure 1: Double-helical structure of actin filaments
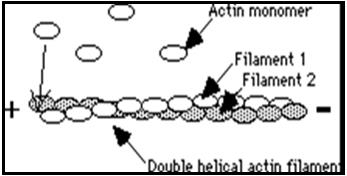
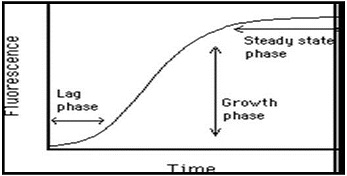
Figure 2: Polymerization of actin as measured by pyrene actin fluorescence
Actin can polymerize from both ends in vitro. However, the rate of polymerization is not equal. This results in an intrinsic polarity in the actin filament. It has therefore become the convention to term the rapidly polymerizing end the plus-end (see Figure 1) or barbed-end while the slow growing end is called the minus-end or pointed-end.
The propensity of actin to polymerize is dependent upon the affinity of actin monomers for filament ends. Thus, there is an actin monomer concentration below which actin will not polymerize. This value has been termed the Critical Concentration (CC). At monomer concentrations above the CC, the actin will polymerize until the free monomer concentration is equal to the CC. When one is working with actin in vitro the extent of actin polymerization depends upon the conditions used. For example, at 4°C muscle actin has a CC of 0.03 mg/ml in the presence of 2 mM Mg2+ and 50 mM KCl, but when these ions are absent, the CC is greater than 3.0 mg/ml. Thus, by altering the ionic type and strength one can alter the amount of polymer formed.
Actin polymerization follows three phases. Just like in microtubule assembly, these are nucleation, growth, and steady state as depicted in Figure 2. The extent of polymerization is indicated by the steady state level of fluorescence. An upper limit can be measured by adding phalloidin (an actin stabilizing compound), which pushes the CC down to <0.01 mg/ml. This assay is greater than 95% accurate and the sample is not disturbed during the assay.
Materials
Purified bovine cardiac muscle actin (Cat. # AD99) has been modified to contain covalently linked pyrene at the cysteine 374 residue. An N-(1-pyrene) iodoacetamide is used to label the actin protein. Pyrene labeling stoichiometry has been determined to be 0.25 dyes per actin monomer. (See Figure 4). Pyrene labeled rabbit muscle actin has an approximate molecular weight of 43 kDa and is supplied as a white lyophilized powder.
Protein purity is determined by scanning densitometry of Coomassie Blue stained protein on a 4-20% polyacrylamide gel. Pyrene muscle actin was found to be >99% pure (see Figure 3).
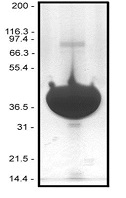
Figure 3. Pyrene Muscle Actin Protein Purity Determination. A 100 µg sample of pyrene muscle actin (molecular weight approx. 43 kDa) was separated by electrophoresis in a 4-20% SDS-PAGE system. The protein was stained with Coomassie Blue. Protein quantitation was determined with the Precision Red Protein Assay Reagent (Cat. # ADV02).
The biological activity of pyrene muscle actin can be determined by its ability to efficiently polymerize into filaments in vitro and separate from unpolymerized components in a spin-down assay. Stringent quality control ensures that > 90% of the labeled muscle actin can be polymerized in this assay.
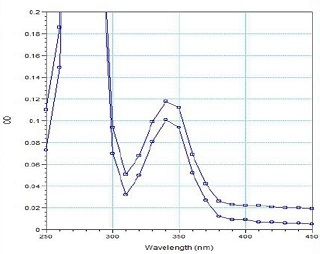
Figure 4. Absorption Scan of pyrene muscle actin in solution. Pyrene muscle actin was diluted in General Actin Buffer and added to a microtiter plate well. Absorbance was scanned between 250 and 500 nm (interval 10 nm), with a pyrene peak at 344nm. Labeling stoich-iometry was calculated via the Beer-Lambert Law to be 0.25 dyes per actin monomer.
Storage
Shipped at ambient temperature. The lyophilized protein can be stored desiccated to <10% humidity at 4°C for 6 months. For reconstitution, briefly centrifuge to collect the product at the bottom of the tube and resuspend each vial to 20 mg/ml with 12.5 µl cold Milli-Q water. The protein will then be in the following buffer: 5mM Tris-HCl pH 8.0, 0.2 mM CaCl2, 0.2 mM ATP, 5% (w/v) sucrose, and 1% (w/v) dextran.
For working concentrations, further dilution of the protein should be made with General Actin Buffer (Cat. # BSA01) supplemented with 0.2 mM ATP (Cat. # BSA04) and 0.5 mM DTT. Pyrene muscle actin is a labile protein and should be handled with care. Diluted pyrene actin is stable for a maximum of 4 h at 4°C and should not be frozen. Avoid repeated freeze-thaw cycles and do not freeze below 20 mg/ml.
Biological Activity Assay
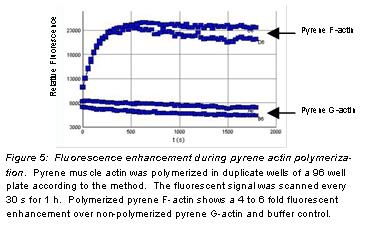
The fluorescent signal of monomer pyrene actin is enhanced during its polymerization into filaments, making it an ideal tool for monitoring actin filament formation. Stringent quality control ensures that pyrene F-actin has a 4-6 fold fluorescent enhancement over non-polymerized pyrene G-actin.
1. Fluorescence Enhancement
Reagents
1. Pyrene Cardiac Muscle Actin (Cat. # CS-AP07)
2. General Actin Buffer (5 mM Tris-HCl pH 8.0, 0.2 mM CaCl2) (Cat. # BSA01)
3. 10 x Polymerization Buffer (500 mM KCl, 20 mM MgCl2, 10 mM ATP) (Cat. # BSA02)
Equipment
1. Fluorescence spectrophotometer with an excitation wavelength of 360 ± 20 nm and an emission wavelength of 405 ± 10 nm or 420 ± 20 nm. Cytoskeleton recommends the SPECTRAFluor Plus from TECAN Austria GmbH or Gemini from Molecular Devices Inc.
2. Black polystyrene 96 well assay plate (Costar, Cat. # 3915).
Method
1. Dilute pyrene muscle actin to 0.30 mg/ml with 0.833 ml General Actin Buffer supplemented with 0.2 mM ATP and 1 mM DTT.
2. Incubate at room temperature for 60 min to depolymerize actin oligomers that form during storage.
3. Centrifuge the protein in a 4°C microfuge at 14k rpm for 30 min to remove residual nucleating centers.
4. Pipette 200 µl of the actin solution into two wells of a black assay 96 well plate.
5. Pipette 200 µl of General Actin Buffer into two wells (control samples).
6. Place the 96 well plate into the fluorescent spectrophotometer and read the samples for 3 min to establish a baseline fluorescent measurement.
7. After 3 min add 20 µl of 10x Actin Polymerization Buffer (Cat. # BSA02) to each well and mix.
8. Return the plate to the spectrophotometer and read the fluorescence every 30 s for 1 h.
9. A typical fluorescent enhancement curve is shown in Figure 5.
Reagents
1. Pyrene Cardiac Muscle Actin (Cat. # CS-AP07)
2. General Actin Buffer (5 mM Tris-HCl pH 8.0, 0.2 mM CaCl2) (Cat. # BSA01)
3. 10 x Polymerization Buffer (500 mM KCl, 20 mM MgCl2, 10 mM ATP) (Cat. # BSA02)
4. 100 mM ATP solution (Cat. # BSA04)
5. 1M DTT solution
6. Precision Red Protein Assay Reagent (Cat. # ADV02)
Equipment
1. Microfuge at 4°C
2. Beckman Airfuge and Ultra-ClearTM centrifuge tubes (Cat. # 344718), Beckman ultracentrifuge and SW 55 Ti rotor with Ultra-ClearTM centrifuge tubes and adapters (Cat. # 358860) or other ultracentrifuge capable of centrifuging 200 µl at 100,000 x g.
3. Spectrophotometer capable of measuring absorbance at 600 nm.
Method
1. Dilute pyrene muscle actin to 0.4 mg/ml in General Actin Buffer supplemented with 0.2 mM ATP and 1 mM DTT.
2. Incubate on ice for 60 min to depolymerize actin oligomers that form during storage.
3. Centrifuge the protein in a 4°C microfuge at 14k rpm for 15 min.
4. Transfer the supernatant to a new microfuge tube and determine the total protein concentration with the Precision Red Protein Assay Reagent.
5. Aliquot 200 µl of the actin dilution to an ultracentrifuge tube.
6. Add 20 µl (1/10th the volume) of 10 x Polymerization Buffer to each airfuge tube and mix well.
7. Incubate at room temperature for 1h.
8. Centrifuge the tubes at 100,000 x g for 1h to pellet the polymerized actin.
9. Remove the top 90% of the supernatant of each tube to a clean microcentrifuge tube.
10. Determine the concentration of the protein in the supernatant (unpolymerized monomer actin) with the Precision Red Advanced Protein Assay Reagent. This protein concentration is used to determined the efficiency with which actin polymerized and pelleted during centrifugation.
Advice for Working with Muscle Actin
1. Monomer actin is unstable in the absence of ATP, a divalent cation and dithiothreitol (DTT).
2. Monomer actin will polymerize at >2 mM K+, Na+, and in >0.05 mM Mg2+.
3. Monomer actin is unstable below pH 6.5, or above pH 8.5.
4. Polymerized actin is more resilient to adverse conditions than monomeric actin. Therefore, actin is preferably stored in the polymerized form at 4°C for several weeks. If filaments are to be stored for longer than 24 h, addition of an antibacterial agent such as 0.05% sodium azide or 100 µg/ml ampicillin and 10 µg/ml chloramphenicol is recommended. Cover in foil to stop photobleaching.
References
1. Holmes K.C., Popp D., Gebhard W. and Kabsch W. 1990. Atomic model of the actin filament. Nature, 347, 44-49.
2. Rayment I., Holden H.M., Whittaker M., Yohn C.B., Lorenz M., Holmes K.C. and Milligan R.A. 1993.Structure of the actin-myosin complex and its implications for muscle contraction. Science, 261, 58-65.
3. Pardo J.V., Pittenger M.F. and Craig S.W., 1983. Subcellular sorting of isoactins: selective association of gamma-actin with skeletal muscle mitochondria. Cell, 32, 1093-1103.
4. Otey C.A., Kalnoski M.H., Lessard J.L. and Bulinski J.C. 1986. Immunolocalization of gamma isoform of nonmuscle actin in cultured cells. Journal of Cell Biology, 102, 1726-1737.
5. Shuster C.B. and Herman I.M. 1995. Indirect association of ezrin with F-actin: Isoform specificity and calcium sensitivity. Journal of Cell Biology, 128, 837-848.
6. Pardee J.D., and Spudich, J.A. 1982. Methods in Cell Biol. 24:271-288.
For product Datasheets and MSDSs please click on the PDF links below.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
Coming soon! If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com





