A Live Look At Membrane Tension At The Plasma Membrane And Beyond
Introduction
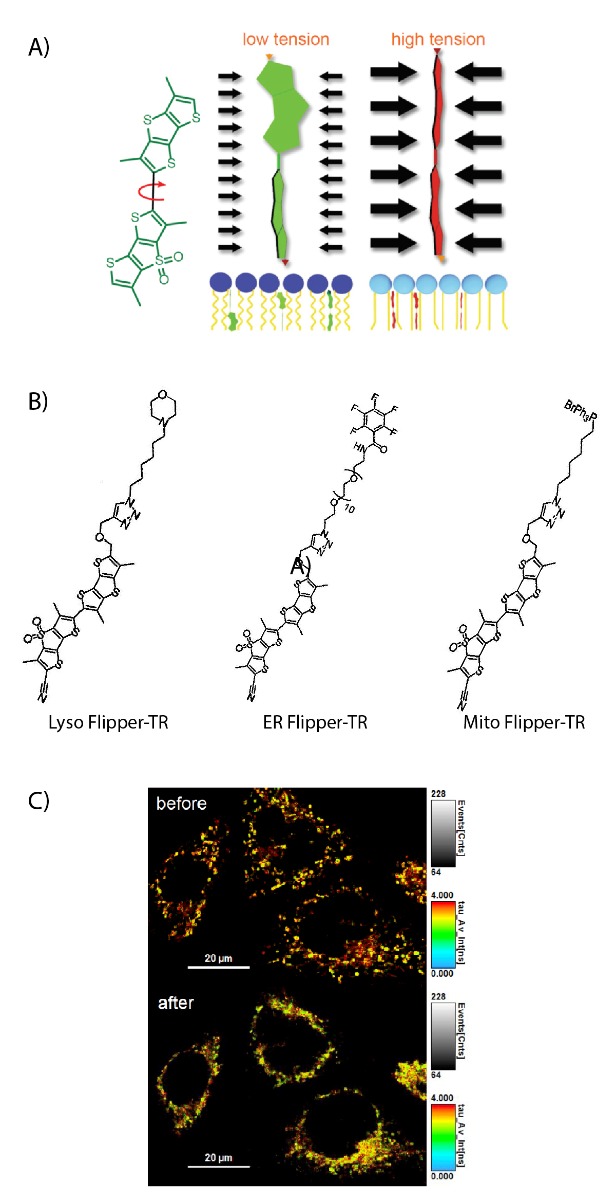
The plasma membrane (PM) is a lipid bilayer that has highly dynamic and fluidic structural properties. These characteristics are essential for cell processes like cell division, migration, and endocytosis. During these cellular processes targeted regions of the PM undergo directed movement which is often carried out by controlled forces from the underlying cytoskeletal network; additionally, mechanical tension, which is the energetic cost of increasing the membrane area, likely plays a role in many of these processes. Scientist’s understanding of membrane tension and the extent to which it controls the aforementioned processes remain incomplete in part due to the challenging and highly specialized techniques used to study it, which include atomic force microscopy and the use of optical tweezers to hold tubes/tethers of the PM(1). A novel planarizable push-pull probe (Figure 1A), Flipper TR, was recently developed, and when paired with Fluorescence-lifetime imaging microscopy it can efficiently measure lipid packing and membrane tension in a linear fashion(2). The following examples highlight how Flipper TR has been used to investigate the role of membrane tension in biological processes as well as in models of disease.
Membrane Tension and Cell Migration
Cell migration is a fundamental process that is critical for development, cancer metastasis, and wound healing amongst many others. While the processes that control protrusion at the front of the cell has been well characterized, the mechanisms dictating retraction at the cell rear remains poorly understood. Established players like non-muscle myosin II, actin filaments, and RhoA are known to play an active role in force generation to retract the rear; however, the mechanisms regulating these proteins are unknown. Recently Hetmanski et al. investigated this process in a 3D matrix model and determined that rear retraction is a dynamic process that involves a front-rear membrane tension differential in fast moving cells(3). In response to low membrane tension, caveolae form at the rear of the cell; in doing so, it activates an Ect2-RhoA-F-Actin axis to promote rear retraction. Localized membrane tension was assessed with Flipper-TR probe and was critical in identifying the role that membrane tension forces play in cell migration.
Figure Legend: A) On the left; schematic image of the Flipper-TR molecule. In the middle a low tension lipid bilayer with Flipper twisted, on the right the probe is planar. B) Schematic image of the Lyso Flipper-TR, ER Flipper-TR, and Mito Flipper-TR molecule. C) FLIM images of HeLa cells labeled with Mito Flipper-TR before and after osmotic shock (Courtesy of Spirochrome).
Membrane Tension and Endocytosis
Endocytosis is the process utilized by eukaryotic cells to internalize substances from their environment, during this process the plasma membrane and associated proteins help to form the vesicle. There is growing evidence that physical forces like membrane tension plays a regulatory role in endocytosis. Recent studies have identified TORC2 as a regulator of endocytosis; interestingly, it utilizes both phosphorylation cascades as well as slower, alternative mechanisms involving changes in the PM biophysical properties(4). In a recent study by Riggi et al. the authors state that TORC2 inhibition leads to enhanced PM tension, which was effectively measured with Flipper-TR(5). This study revealed that TORC2 inhibition impedes endocytosis through two mechanisms one of which is by severing the bonds between PM adaptor proteins, Ent1 and Sla1, and the actin cytoskeleton.
Membrane Tension in Disease Models
The discovery of Flipper-TR has truly expanded the breadth of studies investigating membrane tension and has allowed investigators to implicate membrane tension changes in various disease models. For example, membrane tension enhancement was detected in adipocyte cells exposed to a high-fat diet, which helped to implicate a critical protein, Piezo1, in mediating diet-induced adipogenesis(6). In another study investigating glycolytic activity as a regulator of HIV-1 viral infection, investigators used Flipper-TR to measure host cell membrane tension in response to the glycolysis inhibitor 2-DG and/or cholesterol(7). Another report utilized Flipper-TR to measure nuclear membrane tension to support their findings that mechanical stretch reduces nuclear membrane tension and is an important mechanism to protect the genome against mechanical-stress induced damage(8). In our last example, a recent study identified a VAST domain protein which functions as an autophagy inhibitor and can also monitor membrane tension in the PM through modulation of sterol content(9).
Summary
These examples are but a sample of the growing evidence that membrane tension of the PM plays a regulatory role in critical cellular processes. Recently, a study by Mercier et al. provided evidence that membrane tension of endosomal vesicles is important for vesicle formation, plays a role in endosome trafficking, and responds to EGF induced stimulus to promote intralumenal vesicle formation(10). This study provides strong evidence that membrane tension of organelles can play a regulatory role in organelle form and function. A recent report provides exciting evidence on new Flipper-TR tools that can specifically track membrane tension of the mitochondria, endoplasmic reticulum, and the Lysosome(11). Cytoskeleton Inc. is excited to announce the release of these new Flipper-TR tools to study membrane tension of specific organelles (Figure 1B,C). Undoubtedly, these revolutionary tools will help investigators identify membrane tension mechanisms that regulate specific organelle function.
References
- Sitarska E, Diz-Munoz A. Pay attention to membrane tension: Mechanobiology of the cell surface. Curr Opin Cell Biol. 2020;66:11-8.
- Colom A, Derivery E, Soleimanpour S, Tomba C, Molin MD, Sakai N, et al. A fluorescent membrane tension probe. Nat Chem. 2018;10(11):1118-25.
- Hetmanski JHR, de Belly H, Busnelli I, Waring T, Nair RV, Sokleva V, et al. Membrane Tension Orchestrates Rear Retraction in Matrix-Directed Cell Migration. Dev Cell. 2019;51(4):460-75 e10.
- Rispal D, Eltschinger S, Stahl M, Vaga S, Bodenmiller B, Abraham Y, et al. Target of Rapamycin Complex 2 Regulates Actin Polarization and Endocytosis via Multiple Pathways. J Biol Chem. 2015;290(24):14963-78.
- Riggi M, Bourgoint C, Macchione M, Matile S, Loewith R, Roux A. TORC2 controls endocytosis through plasma membrane tension. J Cell Biol. 2019;218(7):2265-76.
- Wang S, Cao S, Arhatte M, Li D, Shi Y, Kurz S, et al. Adipocyte Piezo1 mediates obesogenic adipogenesis through the FGF1/FGFR1 signaling pathway in mice. Nat Commun. 2020;11(1):2303.
- Coomer CA, Carlon-Andres I, Iliopoulou M, Dustin ML, Compeer EB, Compton AA, et al. Single-cell glycolytic activity regulates membrane tension and HIV-1 fusion. PLoS Pathog. 2020;16(2):e1008359.
- Nava MM, Miroshnikova YA, Biggs LC, Whitefield DB, Metge F, Boucas J, et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell. 2020;181(4):800-17 e22.
- Zhu XM, Li L, Cai YY, Wu XY, Shi HB, Liang S, et al. A VASt-domain protein regulates autophagy, membrane tension, and sterol homeostasis in rice blast fungus. Autophagy. 2020.
- Mercier V, Larios J, Molinard G, Goujon A, Matile S, Gruenberg J, et al. Endosomal membrane tension regulates ESCRT-III-dependent intra-lumenal vesicle formation. Nat Cell Biol. 2020;22(8):947-59.
- Goujon A, Colom A, Strakova K, Mercier V, Mahecic D, Manley S, et al. Mechanosensitive Fluorescent Probes to Image Membrane Tension in Mitochondria, Endoplasmic Reticulum, and Lysosomes. J Am Chem Soc. 2019;141(8):3380-4.
Related Products
Flipper TR™ Plasma Membrane Fluorogenic Probes
Flipper-TR Kit For Fluorescence Cell Membrane Microscopy (Cat. # CY-SC020)
ER Flipper-TR Kit For Fluorescence Cell Membrane Microscopy (Cat. # CY-SC021)
Lyso Flipper-TR Kit For Fluorescence Cell Membrane Microscopy (Cat. # CY-SC022)
Mito Flipper-TR Kit For Fluorescence Cell Membrane Microscopy (Cat. # CY-SC023)
BG Substrates
SPY555-BG Substrate (Cat. # CY-SC204)
SPY620-BG Substrate (Cat. # CY-SC404)
SiR650-BG Substrate (Cat. # CY-SC504)
SiR700-BG Substrate (Cat. # CY-SC604)
Live Cell Imaging Products
SiR-Actin Kit (Cat. # CY-SC001)
SiR-Tubulin Kit (Cat. # CY-SC002)
Cytoskeleton Kit (Includes SiR-Actin, SiR-Tubulin, and Verapamil) (Cat. # CY-SC006)
SiR-Lysosome Kit (Cat. # CY-SC012)
SiR700-Actin Kit (Cat. # CY-SC013)
SiR700-Tubulin Kit (Cat. # CY-SC014)
SiR700-DNA Kit (Cat. # CY-SC015)
SiR700-Lysosome Kit (Cat. # CY-SC016)
Flipper-TR Kit for fluorescence cell membrane microscopy (Cat # CY-SC020)
SPY555-Actin (Cat. # CY-SC202)
SPY555-Tubulin (Cat. # CY-SC203)
SPY650-Tubulin (Cat. # CY-SC503)