Activation Assays
Small G Proteins
Small G-proteins act as molecular switches in a diverse multitude of cellular processes. Distinct from their larger heterotrimeric G protein cousins, small G proteins are monomeric, but share the highly conserved GTPase domain. GTPases cycle between the inactive GDP-bound state and the GTP-bound active state; the latter of which induces a conformational change1. Several small G proteins belonging to the Ras superfamily were characterized as tumor oncoproteins and have received impressive scrutiny by the scientific community due to their cell signaling roles in overrepresented human diseases such as inflammation, neurodegenerations, inflammatory syndrome, and cancer2–4. This newsletter aims to highlight recent Ras superfamily and Rho subfamily discoveries facilitated by Cytoskeleton’s activation assays, which enable investigators to specifically detect the GTP-bound active state of small G proteins.
Ras homolog family member A (RhoA)
The highly conserved RhoA GTPase is a founding member of the Rho GTPase family and has a role in the regulation of numerous biological processes and structures including the actin cytoskeleton5, immunity6, neuronal survival7, and via the promotion of cell motility, cancer8. Dysregulated RhoA expression is associated with worse outcomes in patients with colorectal, hepatic, cervical, and other cancers9–11. In a recent study, Anwar et al. investigated the impact of RhoA-targeting microRNAs (miR, miRNA) to elucidate their impact on colon cancer progression9. To mimic a tumor-like microenvironment HT-29 and AZ-97 cells were cultured in serum-free media. Relative to cells cultured in serum, serum-free cells exhibited increased miRNA-340-5p and decreased RhoA mRNA. Transfection with miR-350-5p reduced Rho mRNA in a dose-dependent manner, in addition to reducing cell migration and cell invasion. A concomitant reduction in protein levels of RhoA-GTP and total RhoA were confirmed with Cytoskeleton’s RhoA G-LISA (Cat. # BK124) and Total RhoA ELISA (Cat. # BK150) kits, respectively. These interesting data suggest that miRNA-340-5p can regulate colon cancer progression by degrading RhoA transcripts.
Rac Family Small GTPase 1 (Rac1)
Rac1 is a p21-Activated kinase (PAK) and plays important roles in cytoskeleton remodeling12, actin dynamics13, neurological development14, and cancers15. Collectively, transformed RAS genes account for nearly a third of all malignancies in humans. To promote cancer cell-survival RAS transformed cancer cells commandeer macropinocytosis, an environment-sampling process normally used by immune cells to combat invading microorganisms, to internalize extracellular proteins and fuel metabolism. In a groundbreaking study, Bhosle et al. elucidated that Slit2 and NSlit2, known to inactivate potent modulators of the actin cytoskeleton, Rac1 and Cdc42, also affect RhoA and in turn regulates macropinocytosis16. Using Cat. # BK124 and Cat. # BK150, investigators observed that NSlit2 increased active-RhoA, but not total RhoA, concomitant with decreased macrophage spread. These changes were reversible by treatment with Rho-specific exoenzyme C3 transferase protein (Cat. # CT03) suggesting RhoA mediates macrophage spread and was also shown to be due to inactivation of MYOB9, which lies downstream of RhoA. An in vitro increase of macropinocytosis was achieved via inhibition of RhoA/B/C with CT03, and reversible via RhoA/B/C activation (Cat. # CN03). CT03-induced enhancement of macropinocytosis was concomitant with increased active Rac levels as observed with Rac1,2,3 G-LISA (Cat. # BK125). Increased Rac1 was abolished with NSlit2 treatment, suggesting inhibition of macropinocytosis is regulated by RhoA activation, and inactivation of Rac1. Altogether these findings reveal that endogenous SLIT proteins are regulators of macropinocytosis that can be driven by GTPase activation.
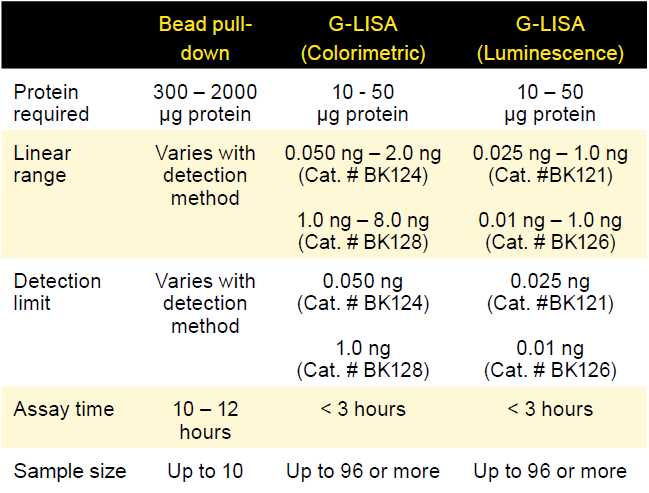
Table 1. Comparison of bead pull-down and G-LISA activation assays
Cell division cycle 42 (Cdc42)
First identified in S. cerevisiae, the highly conserved Cdc42 GTPase is now recognized as having major roles in mammalian biology including determining cell polarity, modulating the actin cytoskeleton, and oncogenic transformation of cells17,18. Although there has been marked improvement in reducing colorectal cancer mortality in recent years, investigators are evaluating alternatives to chemotherapy including the use of anti-tumor oncolytic vaccinia virus (OVV) which selectively lyses cancer cells. In a recent study, Horita et al. aimed to evaluate the impact of host biology on OVV efficacy across several colorectal cancer cell lines using a tripartite-marker panel consisting of long non-coding RNA urothelial carcinoma-associated 1 (UCA1), Cdc42, and filopodia formation19. Results indicate that increased UCA1 expression correlated with OVV-LucGFP spread that was reversible with UCA1 small interfering RNA. Active Cdc42 levels were assessed by colorimetric G-LISA (Cat. # BK127) in 7 cells lines, 4 of which exhibited a clear correlation between enhanced OVV-LG spread and filopodia formation. Taken altogether these data suggest that UCA1, Cdc42 activation, and filopodia morphology comprise a robust three-panel marker for OVV spread.
ADP-ribosylation factor (ARF)
Arf small GTPases belong to the Arf subfamily and are best known for regulating vesicular traffic and actin cytoskeleton remodeling20,21. Arf6 has been shown to interact with other small G-proteins like Rac1 to regulate process like actin remodeling. While dysregulation of the small G-protein Rac1 has previously been identified in podocytes and associated with kidney damage, the role of Arf6 in podocyte cells was largely unknown. In a recent study, Lin et al. used the Arf6 bead pull-down kit (Cat. # BK033-S) and provided the first evidence of inducible Arf6-GTP in wild-type (WT) mice using a nephrin tyrosine phosphorylation-induced podocyte-effacement model22. Stimulation of podocyte signaling in WT podocytes by nephrin resulted in significantly elevated levels of Arf6 activity and association with nephrin. Nephrin treatment also elevated Rac1-GTP (Cat. # BK128) in WT podocytes, but conversely, Arf6 knockdown mutants exhibited an overall decrease of active Rac1. These data suggest a Rac1-Arf6 mediated mechanism underlies kidney injury. In a related study, Che et al. interrogated immortalized human podocytes and discovered that active Arf6 levels were significantly elevated (Cat. # BK133) concomitant with elevated reactive oxygen species (ROS) and apoptosis following Angiotensin II treatment (Ang II)23. Although Arf6’s role in Ang II-induced podocyte damage is documented, the molecular mechanisms behind this pathology are not well known. Importantly, these effects were linked to a degradation of CD2-associated protein (CD2AP). Rescue of CD2AP was able to inhibit active-Arf6 and significantly reduce ROS and apoptosis suggesting that Arf6 inhibition via CD2AP rescue may alleviate Ang II-induced podocyte injury.
Summary
As highlighted in these examples, Cytoskeleton's activation assays are empowering tools to discover novel small G-proteins roles, interactions, or complex molecular relationships in cell signaling events. To help scientists continue in their groundbreaking GTPase studies, Cytoskeleton offers RAS superfamily activation assays in two formats each with distinct advantages. The G-LISA format utilizes 96-well strip plates, are ideally suited for rare samples (Table 1), can be performed in ~3 hours, and produce quantitative results. Pull-down kits also have their own distinct advantages such as economical costs, utilization of standard immunoprecipitation techniques, and only requires ubiquitous western blot supplies. Both activation assay formats are highly cited and available to help scientists with their next novel small G-protein discovery.
References
- Nestler, E. J. & Duman, R. S. Small G Proteins. (1999).
- Colicelli, J. Human RAS superfamily proteins and related GTPases. Science’s STKE : signal transduction knowledge environment vol. 2004 RE13 (2004).
- Sastre, A. A. et al. Small gtpases of the ras and rho families switch on/off signaling pathways in neurodegenerative diseases. International Journal of Molecular Sciences vol. 21 1–23 (2020).
- Catanzaro, J. M. et al. Oncogenic Ras induces inflammatory cytokine production by upregulating the squamous cell carcinoma antigens SerpinB3/B4. Nat. Commun. 5, 1–12 (2014).
- Tkach, V., Bock, E. & Berezin, V. The role of RhoA in the regulation of cell morphology and motility. Cell Motil. Cytoskeleton 61, 21–33 (2005).
- Bros, Haas, Moll & Grabbe. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells 8, 733 (2019).
- Hu, J. & Selzer, M. E. RhoA as a target to promote neuronal survival and axon regeneration. Neural Regeneration Research vol. 12 525–528 (2017).
- Shimokawa, H., Sunamura, S. & Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circulation Research vol. 118 352–366 (2016).
- Algaber, A. et al. MicroRNA-340-5p inhibits colon cancer cell migration via targeting of RhoA. Sci. Rep. 10, 1–8 (2020).
- Tanaka, K. et al. Impact of RhoA overexpression on clinical outcomes in cervical squamous cell carcinoma treated with concurrent chemoradiotherapy. J. Radiat. Res. 61, 221–230 (2020).
- Jeong, D. et al. RhoA is associated with invasion and poor prognosis in colorectal cancer. Int. J. Oncol. 48, 714–722 (2016).
- Yuki, K. E. et al. CYRI/FAM49B negatively regulates RAC1-driven cytoskeletal remodelling and protects against bacterial infection. Nat. Microbiol. 4, 1516–1531 (2019).
- Watson, J. R., Owen, D. & Mott, H. R. Cdc42 in actin dynamics: An ordered pathway governed by complex equilibria and directional effector handover. Small GTPases vol. 8 237–244 (2017).
- Linseman, D. A. & Loucks, F. A. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Frontiers in Bioscience vol. 13 657–676 (2008).
- Li, Q. et al. Rac1 activates non-oxidative pentose phosphate pathway to induce chemoresistance of breast cancer. Nat. Commun. 11, 1–18 (2020).
- Bhosle, V. K. et al. SLIT2/ROBO1-signaling inhibits macropinocytosis by opposing cortical cytoskeletal remodeling. Nat. Commun. 11, 1–17 (2020).
- Stengel, K. & Zheng, Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cellular Signalling vol. 23 1415–1423 (2011).
- Feng, Q. & Cerione, R. A. Cdc42 and its cellular functions. in Handbook of Cell Signaling, 2/e vol. 2 1785–1794 (Elsevier Inc., 2010).
- Horita, K. et al. Long noncoding RNA UCA1 enhances sensitivity to oncolytic vaccinia virus by sponging miR-18a/miR-182 and modulating the Cdc42/filopodia axis in colorectal cancer. Biochem. Biophys. Res. Commun. 516, 831–838 (2019).
- Boshans, R. L., Szanto, S., van Aelst, L. & D’Souza-Schorey, C. ADP-Ribosylation Factor 6 Regulates Actin Cytoskeleton Remodeling in Coordination with Rac1 and RhoA. Mol. Cell. Biol. 20, 3685–3694 (2000).
- D’Souza-Schorey, C. et al. ARF6 targets recycling vesicles to the plasma membrane: Insights from an ultrastructural investigation. J. Cell Biol. 140, 603–616 (1998).
- Lin, J. S. et al. ARF6 mediates nephrin tyrosine phosphorylation-induced podocyte cellular dynamics. PLoS One 12, (2017).
- Che, G., Gao, H., Hu, Q., Xie, H. & Zhang, Y. Angiotensin II promotes podocyte injury by activating Arf6-Erk1/2-Nox4 signaling pathway. PLoS One 15, e0229747 (2020).
Related Products
Bead pull-down Activation Assays
Arf1 Activation Assay Biochem Kit (Cat. # BK032-S)
Arf6 Activation Assay Biochem Kit (Cat. # BK033-S)
GGA3-PBD Beads (Arf1 + Arf6) (Cat. # GGA07)
Cdc42 Activation Assay Biochem Kit (Cat. # BK034)
Rac + Cdc42 Activation Assay Biochem Kit (Cat. # PAK02)
Rac1 Activation Assay Biochem Kit (Cat. # BK035)
Ras Activation Assay Biochem Kit (Cat. # BK008)
Rhotekin RBD beads (RhoA/B/C) (Cat. # RT02)
RhoA Activation Assay Biochem Kit (Cat. # BK036)
RhoA/Rac1/Cdc42 Activation Assay Combo Biochem Kit (Cat. # BK030)
Actin Biochem Kits
Arf1 G-LISA™ Activation Assay Kit (Colorimetric format) (Cat. # BK132)
Arf6 G-LISA™ Activation Assay Kit (Colorimetric format) (Cat. # BK133)
Cdc42 G-LISA™ Activation Assay Kit (Colorimetric format) (Cat. # BK127)
Rac1 G-LISA™ Activation Assay Kit (Luminescence format) (Cat. # BK126)
Rac1 G-LISA™ Activation Assay Kit (Colorimetric format) (Cat. # BK128)
RalA G-LISA™ Activation Assay Kit (Colorimetric format) (Cat. # BK129)
RhoA G-LISA™ Activation Assay (Luminescence format) (Cat. # BK121)
RhoA G-LISA™ Activation Assay Kit (Colorimetric format) (Cat. # BK124)
RhoA / Rac1/ Cdc42 G-LISA™ Activation Assay Bundle 3 kits (24 assays per kit) (Cat. # BK135)
Rac1,2,3 G-LISA™ Activation Assay (Colorimetric format) (Cat. # BK125)
Rac1 G-LISA™ Activation Assay (Luminescence format) (Cat. # BK126)
Rac1 G-LISA™ Activation Assay Kit (Colorimetric Based) (Cat. # BK128)
Ras G-LISA™ Activation Assay Kit (Colorimetric Based) (Cat. # BK131)
Total RhoA ELISA (Cat. # BK150)