Live Cell Imaging and CNS Diseases and Disorders
Studying the causes of human central nervous system (CNS) diseases and disorders and developing impactful therapies requires in vitro and in vivo disease models that faithfully recapitulate the respective neuropathophysiology while also supporting the neurons with the necessary cellular machinery to respond to therapies in a manner that offers translational results1-3. Moreover, it is imperative to study the earliest neuropathophysiological changes as these provide the best opportunity for early diagnosis and intervention4. Changes in the structure and/or function of neuronal synapses are often the earliest pathological changes in many neurodegenerative diseases and CNS disorders4-7. To detect early synaptic dysfunction, scientists utilize live cell imaging which provides single cell resolution with real-time analysis of changing experimental conditions. This newsletter discusses the use of live cell imaging in in vitro and in vivo studies of CNS diseases and disorders.
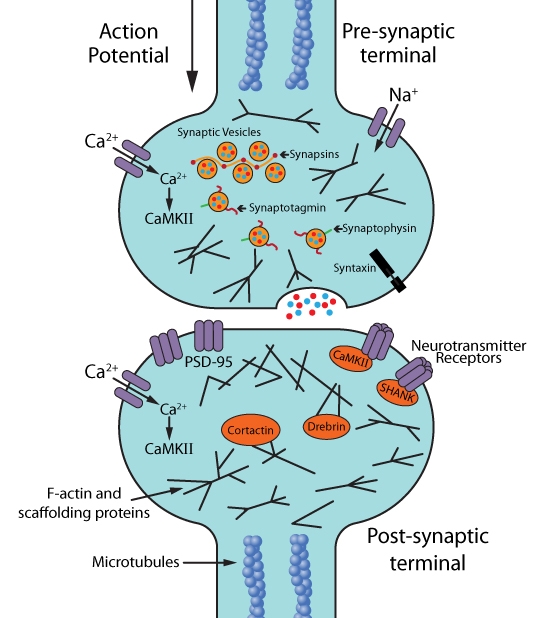
Designing biologically-relevant in vitro and in vivo live cell imaging experiments requires healthy, mature neurons with proper synaptic structure and function (i.e., morphology and electrical activity, respectively) (Fig. 1). Primary imaging targets are pre- and post-synaptic structures and associated proteins (e.g., synaptic vesicle proteins, neurotransmitter receptors, ion channels, scaffolding proteins, and/or cytoskeletal proteins [F-actin, microtubules]) because they are among the earliest substrates to undergo pathological changes4 (Fig. 1). Methods to assess changes in synaptic structure and/or function include measuring synapse density, neurite length, spine density, nodes (the location on the cell body from which neurites project), branching of dendrites, and/or electrical activity4.
Live cell imaging of in vitro cultured mammalian neurons, including human-induced pluripotent stem cell (hiPSC)-derived neurons utilized due to their presumed greater biological relevance8, has revealed unique mechanistic and functional findings relevant to human neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Apolipoprotein E (apoe-E) is a protein implicated in the pathogenesis of AD, but the role of apoe-E and its metabolites in neuron physiology is unappreciated. Differentiated SH-SY5Y neuroblastoma cells chronically treated with a 25 kDa fragment or full-length apoE display neuritogenesis (positive changes in neurite length and cell confluency as assessed by live cell imaging)9. Preliminary drug screening studies also benefit from this methodology. In AD, the two most common drug targets are amyloid beta (Aβ) and tau proteins as both of these proteins have early pathological effects on the synapse5-7. Recently, live cell imaging of hiPSC-derived neurons was performed in the screening of several candidate antibodies against pathological Aβ. Positive immunotherapeutic findings were assessed by quantifying changes in neurite length and dendritic branch points over a range of antibody incubation times and concentrations10. In another Aβ study, Hong et al.11 imaged living hiPSC-derived neurons treated with Aβ oligomers isolated from post-mortem AD human brain extracts. The pathophysiology of the oligomers was assessed through quantification of changes in neurite length and synaptic plasticity with long-term potentiation recordings. Tau protein is the focus of a novel technology with the creation of the first genetically encoded Foster Resonance Energy Transfer (FRET) sensor sensitive to tau conformations. The sensor monitors the conformations of wild-type and pathological mutant tau proteins in the presence and absence of microtubules (MTs) in living HeLa cells and immortalized HT22 hippocampal neurons following pharmacological treatments12. This tau FRET sensor provides invaluable quantitative and qualitative data concerning modulation of tau binding to MTs, the ratios of soluble to insoluble (pathological) tau, and/or how to influence tau’s conformation to favor non-pathological forms.
The complement to in vitro cell culture models is in vivo animal models. Live cell imaging of neurons in whole animals is often performed with two photon microscopy which allows observation of functional neurons with single cell resolution in their natural state and within the larger setting of an interconnected support network13. In this way, live cell imaging in animals is transformative with the opportunity to visualize and analyze dynamic cellular processes in real-time. Additionally, single cell in vivo imaging allows for quantification of neuronal functional responses concomitant with sensory input, behavior, and/or pharmacological treatments13.
In vivo two photon live cell imaging was utilized in the hunt for the mechanism of action of an anti-PD drug that targets α-synuclein14 and for evaluating the feasibility and optimal timing, host, and donor conditions for transplanting embryonic cells into damaged adult brain regions to replace dead or dying neurons15-17. Moreover, in vivo imaging can reveal new therapeutic approaches. In the MPTP mouse model of PD, live cell imaging showed that the structural and functional synaptic plasticity of motor cortical neurons was affected by the selective loss of dopaminergic neurons in MPTP-treated mice, raising the possibility that modulating neuronal activity in particular regions of motor cortex is a viable option for treating the motor impairments associated with PD18.
Summary
Live cell imaging is an invaluable tool in the study of CNS diseases and disorders as it offers insight into not only the basic disease/disorder biology, but also the functional consequences of pharmacological, genetic, and/or behavioral therapy interventions in real time. When coupled with advances in super resolution microscopy and automated live cell imaging19, there are unparalleled opportunities to focus on the first pathological synaptic changes that can be halted or reversed with early therapeutic intervention. At Cytoskeleton Inc., valuable research tools such as live cell imaging probes for F-actin, microtubules, DNA, and lysosomes are available, as well as purified cytoskeletal proteins and activation assays and antibodies, to advance these research goals.
References
- Neurological disease models made clear. Nat. Med. (Editorial, published September 2015). 21, 964.
- Schlachetzki J.C. et al. 2013. Studying neurodegenerative diseases in culture models. Rev. Bras. Psiquiatr. 35, S92-100.
- Hughes P. et al. 2018. The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? Biotechniques. 43, 575, 577-578, 581-582
- Verstraelen P. et al. 2018. Image-based profiling of synaptic connectivity in primary neuronal cell culture. Front. Neurosci. 12, 389.
- Hoover B.R. et al. 2010. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 68, 1067-1081
- Spires-Jones T.L. and Hyman B.T. 2014. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 82, 756-771.
- Bayer T.A. and Wirths O. 2010. Intracellular accumulation of amyloid-beta – a predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front. Aging Neurosci. 2, 8
- Unternaehrer J.J. and Daley G.Q. 2011. Induced pluripotent stem cells for modelling human diseases. Phil. Trans. R. Soc. B. 366, 2274-2285.
- Munoz S.S. et al. 2018. The serine protease HtrA1 contributes to the formation of an extracellular 25-kDa apolipoprotein E fragment that stimulates neuritogenesis. J. Biol. Chem. 293, 4071-4084
- Jin M. et al. 2018. An in vitro paradigm to assess potential anti-Aβ antibodies for Alzheimer’s disease. Nat. Commun. 9, 2676.
- Hong W. et al. 2018. Diffusible, highly bioactive oligomers represent a critical minority of soluble Aβ in Alzheimer’s disease brain. Acta Neuropathol. 136, 10-40
- Di Primio C. et al. 2017. The distance between N and C termini of tau and of FTDP-17 mutants is modulated by microtubule interactions in living cells. Front. Neurosci. 10, 210.
- White M.D. et al. 2018. In vivo imaging of single mammalian cells in development and disease. Trends Mol. Med. 24, 278-293.
- Wrasidlo W. et al. 2016. A de novo compound targeting alpha-synuclein improves deficits in models of Parkinson’s disease. Brain. 139, 3217-3236.
- Falkner S. et al. 2016. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature. 539, 248-253.
- Peron S. et al. 2017. A delay between motor cortex lesions and neuronal transplantation enhances graft integration and improves repair and recovery. J. Neurosci. 37, 1820-1834.
- Wang C. et al. 2016. Infiltrating cells from host brain restore the microglial population in grafted cortical tissue. Sci. Rep. 6, 33080.
- Guo L. et al. 2015. Dynamic rewiring of neural circuits in the motor cortex in mouse models of Parkinson’s disease. Nat. Neurosci. 18, 1299-1309.
- Shin H.Y. et al. 2018. Using automated live cell imaging to reveal early changes during human motor neuron degeneration. eNeuro. doi: 10.1523/ENEURO.0001-18.2018.
Related Products
Live Cell Imaging Products
SiR-Actin Kit (Cat. # CY-SC001)
SiR-Tubulin Kit (Cat. # CY-SC002)
Cytoskeleton Kit (Includes SiR-Actin, SiR-Tubulin, and Verapamil) (Cat. # CY-SC006)
SiR-Lysosome Kit (Cat. # CY-SC012)
SiR700-Actin Kit (Cat. # CY-SC013)
SiR700-Tubulin Kit (Cat. # CY-SC014)
SiR700-DNA Kit (Cat. # CY-SC015)
SiR700-Lysosome Kit (Cat. # CY-SC016)
G-LISA Activation Assay Kits
G-LISA RhoA Activation Assay Biochem Kit (colorimetric format) (Cat. # BK124)
G-LISA RhoA Activation Assay Biochem Kit (luminescence format) (Cat. # BK121)
Actin Biochem Kits
Actin Binding Protein Spin-Down Assay Biochem Kit: rabbit skeletal muscle actin (Cat. # BK001)
Actin Binding Protein Spin-Down Assay Biochem Kit: human platelet actin (Cat. # BK013)
Actin Polymerization Biochem Kit (fluorescence format): rabbit skeletal muscle actin (Cat. # BK003)
G-Actin/F-actin In Vivo Assay Biochem Kit (Cat. # BK037)
Tubulin Biochem Kits
Actin Binding Protein Spin-Down Assay Biochem Kit: rabbit skeletal muscle actin (Cat. # BK006P)
Actin Binding Protein Spin-Down Assay Biochem Kit: human platelet actin (Cat. # BK011P)
Actin Polymerization Biochem Kit (fluorescence format): rabbit skeletal muscle actin (Cat. # BK029)