Live Cell Imaging Citation Spotlights
Citation Spotlight 12/8/20: Amyloid beta-induced F-actin destabilization alters dendritic spines in models of Alzheimer’s disease
Citation Spotlight 11/3/20: MICOS controls mitochondrial cristae formation
Citation Spotlight 4/28/20: Porcine Hemagglutinating Encephalomyelitis Virus Orchestrates Cytoskeletal and Transcriptional Changes of Host Neurons to Support Invasion
Citation Spotlight 1/2/20: Arp2/3 regulates RhoA activity and protein levels through cortactin/p190RhoGAP and CCM2/Smurf1, respectively
Citation Spotlight 1/2/20: Xenopus Egg Cytoplasm Self-assembles into Spatially Organized, Cell-like Compartments De Novo
Citation Spotlight 12/3/19: Transient Calcium Waves, Actin Dynamics, and Wound Healing
Citation Spotlight 10/21/19: Polyglutamylation Regulates Motility of Neuron-specific KIF1A Kinesin
Citation Spotlight 11/12/18: Tctex-1, Novel Binding Partner of KIM-1, and Phagocytosis of Apoptotic Cells
Citation Spotlight 8/27/18: Misshapen Kinase Alters Actin Dynamics in the Regulation of Ring Canal Size and Stability
Citation Spotlight 5/15/18: Regulating RhoA Activity by Modulating RhoGDI Alpha Post-Translational Modifications
Citation Spotlight 3/6/18: Actin Bundling Protein EFhd2 Mediates LPS-Induced Macrophage Migration
Citation Spotlight 1/3/18: Acetylated Microtubule Bundling, MAP Binding, and Functional Regulation of Kinesin-1
Citation Spotlight 12/18/17: Anti-cancer Therapeutic Buparlisib: Mitosis or PI3K Inhibitor?
Citation Spotlight 6/14/17: Fibronectin Recycling and Fibrillogenesis Regulation by Transforming Growth Factor ß
Citation Spotlight 2/21/17: Characterization of Novel 3D In Vitro Model of Cancer Cell Invasion
Citation Spotlight 12/21/16: Rho-Family GTPase Activity: Key Components of Anti-Inflammatory and Neuroprotective Pathway
Citation Spotlight 2/2/16: Cdc42 Activity Mediates Podoplanin-induced Tumor Progression
For more specific information about our Live Cell Imaging products please view our product pages and datasheets.

Citation Spotlight: Amyloid beta-induced F-actin destabilization alters dendritic spines in models of Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder manifest by impaired cognitive function and memory loss. Various changes in neuron morphology underlie AD’s clinical symptoms including synapse loss; in mouse models of AD this is represented via a loss of dendritic spines. Dendritic spines are enriched with helical filamentous-actin (F-actin), a predominant cytoskeleton protein, and undergo remodeling for the stabilization of memories after learning. A study by Kommadi et al. investigated whether increased pools of G-actin and decreased F-actin preceded AD onset or were a consequence of dendritic spine loss. Relative to WT littermate controls, synaptosomes in mouse models of AD (APP/PS1) displayed diminished levels of F-actin and increased G-actin, despite not having a decrease in total actin nor displaying pathologic hallmarks of the disease (Cat. # BK037). Pyrene-actin assays (Cat. # BK003) revealed that actin in the presence of Aβ1-42 peptide displayed disrupted polymerization kinetics similar to APP/PS1 actin polymerization. Long-term in vitro staining of primary cortical neurons with DiI or phalloidin (Cat. # PHDG1) revealed that a reduction of F-actin was present at day 10 in APP/PS1 neurites without concomitant change in dendritic spine number or surface area. At day 16 a marked decrease of spines, spine dendrites, and spine surface area was observed in APP/PS1 neurons, concomitant with a significant reduction of F-actin in tertiary neurites. Primary cortical neurons stained with phalloidin and treated with Ab42 exhibited dose-dependent diminishment of F-actin, suggesting amyloid-β can depolymerize F-actin. APP/PS1 mice displayed a reduced fear conditional potential relative to WT controls, that was reversible with actin-stabilizing jasplakinolide, suggesting disruption of F-actin perturbs memory formation. Relative to wild-type neurons, APP/PS1 mushroom spine heads labeled with phalloidin or DiI were quantified with dSTORM and exhibited significantly reduced actin rod length and order arising from altered F-actin architecture. Examination of G:F actin ratios (Cat. # BK037) in post-mortem synaptosomes from humans with no cognitive impairment (NCI), mild cognitive impairment (MCI), and AD revealed significantly decreased F-actin without change in G-actin. A significant correlation was also found between reduced F-actin and diminished global cognition, β-amyloid levels, neurofibrillary tangles, and Braak staging. Cytoskeleton’s actin tools highlighted above played an essential role in revealing the importance that F-actin has in AD progression and it will be interesting to further unravel the interaction between actin and AD.
Products Used:
Actin Polymerization Biochem Kit (fluorescence format): rabbit skeletal muscle actin (Cat. # BK003)
G-Actin/F-actin In Vivo Assay Biochem Kit (Cat.# BK037)
Related Products:
Acti-stain™ 535 (Cat. # PHDR1)
Acti-stain™ 555 (Cat. # PHDH1)
Acti-stain™ 670 (Cat. # PHDN1)
MemGlow™ 488: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG01))
MemGlow™ 560: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG02)
MemGlow™ 590: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG03)
MemGlow™ 640: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG04)
MemGlow™ 700: Fluorogenic Membrane Probe (a member of the MEMBRIGHT™ family) (Cat. # MG05)
SPY555-actin (Cat. # CY-SC202)
SiR700-Actin Kit (Cat. # CY-SC013)
SiR-Actin Kit (Cat. # CY-SC001)
Anti-Pan Actin Mouse Monoclonal Antibody (Clone 7A8.2.1) (Cat. # AAN02)
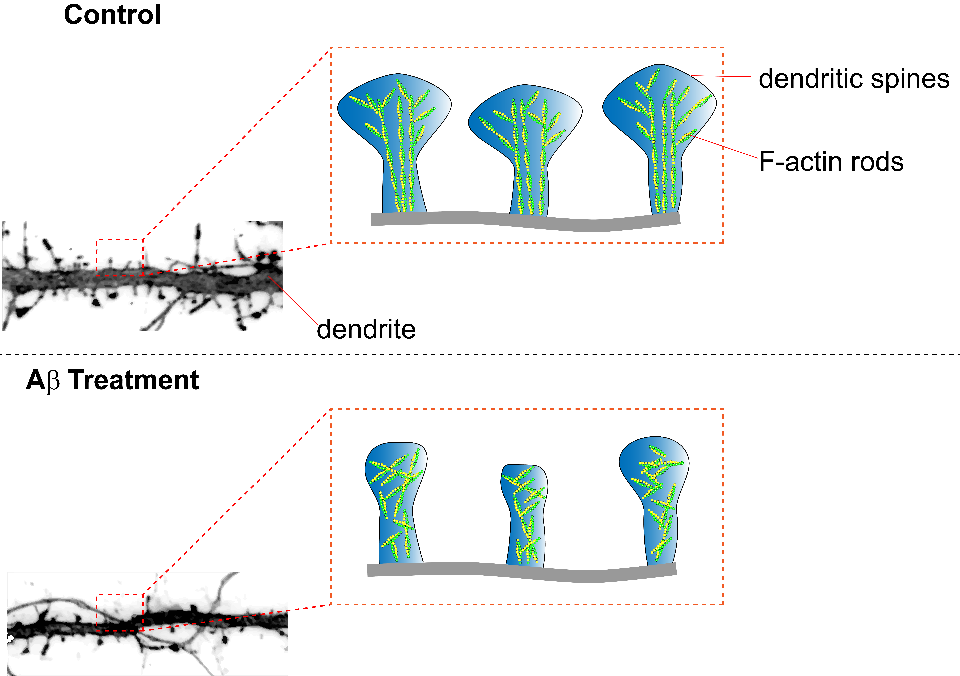
Caption 1: Summary figure illustrating the effect of amyloid beta treatment on neurons. Dendritic spines treated with amyloid beta peptides exhibit decreased dendritic spine surface area, F-actin rod length, and anisotropy (parallel arrangement of F-actin rods).
Citation Spotlight: MICOS controls mitochondrial cristae formation
Mitochondria are the chief power plants for eukaryotic cells and generate ATP through oxidative phosphorylation. Many debilitating and lethal disease are associated with abnormal cristae morphologies in the mitochondria; however, a complete elucidation of cristae morphology-directing complexes such as the Mitochondrial contact site and Cristae Organizing System (MICOS) is lacking. Stephan et al. used extensive microscopy and molecular biology tools to identify the distinct roles that Mitochondrial Dynamin Like GTPase (OPA1), F1FO-ATP synthase, and MICOS subunits Mic60 and Mic10 exert on cristae biogenesis and overall mitochondrial morphology. To characterize the nominal structure of HeLa mitochondria, SNAP-tagged chrome c oxidase subunit 8A (COX8A) conjugated to SiR-BG was employed. Investigators observed a predominance of lamellar cristae interspaced by compartments containing mitochondrial nucleoids, and an overall structural heterogeneity of the mitochondrial inner membrane. Systematic CRISPR/Cas9 knockout of MICOS subunits was used to identify the roles of MICOS subunits, including Mic60, which also resulted in a near complete reduction of Mic10, 13, 19, and 26 with incomplete yet strongly reduced levels of 25 and 27. Knockout of Mic19 recapitulated these findings while knockout of Mic10 or 13 did not; although, loss of these Mics were associated with a strong reduction in other Mic subunits. These data suggest that specific subunits, such as Mic60, play central roles in the formation of MICOS. Morphologically, Mic60-KO cells exhibited fragmented mitochondrial networks that were spherically shaped and displayed highly disordered cristae. Modulation of Mic10 levels revealed that the distribution of Mic60 is linked to Mic10 levels in a concentration-dependent manner, and relative to wild-type, affected the overall organization of the mitochondria. The loss of Mic10 and/or Mic60 resulted in severely aberrant cristae membranes appearing onion-shaped or longitudinal, respectively, rather than the perpendicular projections, suggesting that Mic10 and 60 together control cristae architecture. The inner mitochondrial membrane contributes to the shape of cristae and is influenced by F1Fo-ATP synthase dimers. When dimer-forming ATP5ME, a subunit of F1Fo-ATP synthase, was perturbed through RNAi, mitochondria exhibited a reduced number of lamellar cristae with only a modest change in morphology; however, Mic60 distribution was altered. This suggests F1Fo-ATP synthase works chiefly in concert with Mic60 to stabilize cristae architecture. OPA1-deficient mitochondria also appeared to influence Mic10, with knock-on effects that influenced the distribution of Mic60, appearing to work in conjunction with Mic10 to stabilize tubular CJs. Altogether, these data suggest that mitochondrial cristae biogenesis is heavily shaped by a complex interplay between MICOS, F1FO-ATP synthase, and OPA1. BG-SiR was an essential reagent in this study as investigators sought to identify the localization of Mic subunits in the mitochondria. Cytoskeleton offers several fluorogenic, cell permeable BG-SPY substrates for these types of applications.
Note: SiR650-BG (Cat. # CY-SC504) is identical to the substrate used to label Mic10-SNAP and COX8A-SNAP.
Link to the Citation:
Products Used:
Directly Related Products:
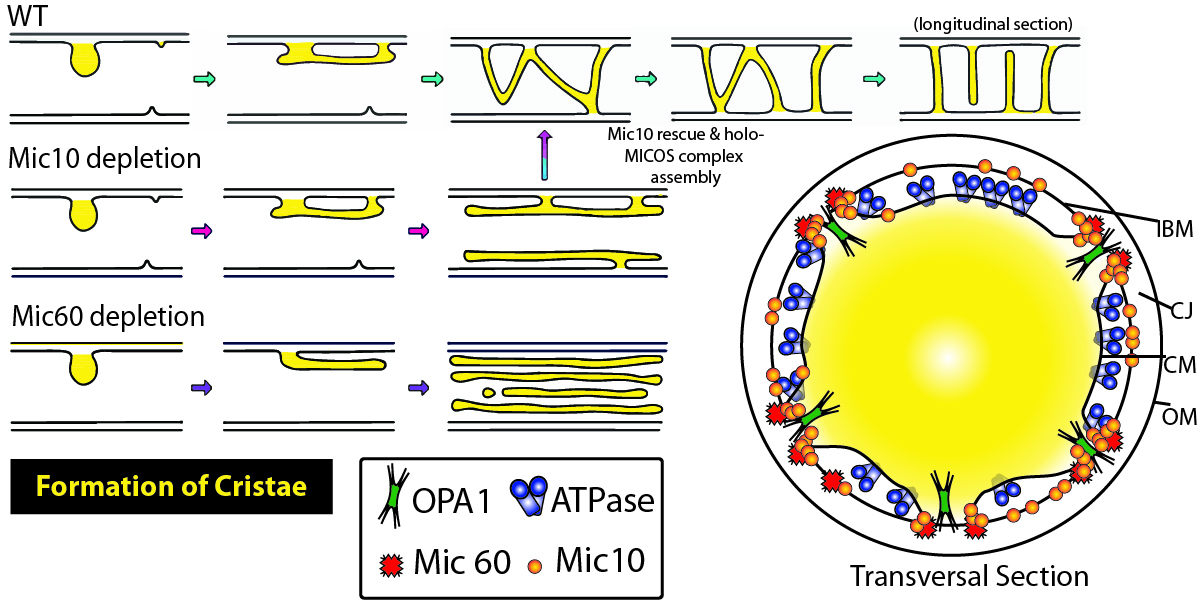
Caption 1: Summary figure illustrating the contribution of Mic10 and Mic60 subunits in cristae morphology and consequences of their loss. Inner boundary membrane (IBM), cristae junction (CJ), cristae membrane (CM), outer membrane (OM), Mitochondrial Dynamin Like GTPase (OPA1), Mitochondrial contact site and Cristae Organizing System (MICOS).
Citation Spotlight: Porcine Hemagglutinating Encephalomyelitis Virus (PHEV) Orchestrates Cytoskeletal and Transcriptional Changes of Host Neurons to Support Invasion
Porcine Hemagglutinating Encephalomyelitis Virus (PHEV) is an enveloped neurotropic single-stranded RNA coronavirus, belonging to the family Coronaviridae and genus Betacoronavirus, that causes profound neurological symptoms in pigs. PHEV infection entails the removal of key barriers to entry including the temporary dissolution or remodeling of protective cortical actin. In this study authors thoroughly interrogated the mechanism of PHEV infection on N2a cells and its subsequent cellular consequences. Investigators found PHEV infection was followed by actin cytoskeleton remodeling that included vacillating abundance of stress fibers, filopodia, and lamellipodia. Phosphorylation analysis of cofilin, a regulator of filamentous actin, revealed a link between actin remodeling and cofilin activation. Modulating actin remodeling by transfecting constitutively active, inactive, or wild-type cofilins resulted in attenuated PHEV penetration. Similarly, CytochalasinD-induced collapse of the cytoskeleton reduced viral titers and taken altogether suggests that functional actin and cytoskeletal regulation through biphasic cofilin activation is required for successful infection. When upstream factors of cofilin regulation were pharmacologically interrogated via ATN-161-induced inhibition of integrin α5β1, and PF-573228-induced inhibition of non-receptor tyrosine protein kinase FAK, viral entry was significantly inhibited concomitant with cofilin phosphorylation suggesting that PHEV binding to integrin α5β1 induces FAK-signaling that in turn promotes PHEV infiltration. Given the influence GTPases exert on actin dynamics the authors then inhibited Rac1 and Cdc42 with EHoP-016 and ML-141, respectively, which impacted cofilin phosphorylation during invasion and subsequently inhibited viral entry. Furthermore, a pulldown assay revealed that chronological activation of Rac1 and Cdc42 corresponded with cofilin phosphorylation and actin dynamics during infection, and this effect was reversible by ATN-161 and PF-573228. LIM Domain Kinase (LIMK) and p21-activated kinase PAK1, which reside downstream of Rac1 and Cdc42, were shown to be phosphorylated during early infection. When PAK1, which phosphorylates LIMK, was selectively inhibited via IPA-3 PHEV-induced formation of filopodia and lamellipodia, as well as cofilin phosphorylation and viral entry were inhibited. The authors conclude that PHEV infection initiates viral entry through an integrin α5β1-FAK-Rac1/Cdc42-PAK-LIMK signaling pathway that elicits cofilin-induced actin remodeling required for viral entry and trafficking. The majority of the interrogations of viral invasion found in this study were conducted with microscopical visualization and quantification, as well as flow cytometry quantification, and greatly enhanced by Cytoskeleton’s fluorescently conjugated phalloidin product Acti-stain 488™ (Cat.# PHDG1).
Note: The authors unintentionally refer to the labeled phalloidin as FITC-phalloidin; however, based on our product lineup we conclude that they are referring to Acti-stain 488™ which uses a proprietary dye distinct from FITC.
Products Used in this Citation:
Directly Related Products:
Acti-stain™ 535 (Cat. # PHDR1)
Acti-stain™ 555 (Cat. # PHDH1)
Acti-stain™ 670 (Cat. # PHDN1)
Cdc42 Pull-down Activation Assay Biochem Kit (bead pull-down format) - 50 Assays (Cat. # BK034)
Rac1 Pull-down Activation Assay Biochem Kit (bead pull-down format) - 50 Assays (Cat. # BK035)
Rac1 G-LISA Activation Assay (Luminescence format) - 96 assays (Cat. # BK126)
Cdc42 G-LISA Activation Assay (Colorimetric format) - 96 assays (Cat. # BK127)
Rac1 G-LISA Activation Assay Kit (Colorimetric Based) 96 assays (Cat. # BK128)
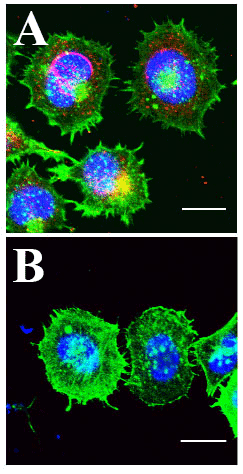
A) HCT-8 Cells Infected with PHEV coronavirus. Actin labeled with Cytoskeleton Acti-stain 488 (green), PHEV coat protein antibody labeled (red) and DNA with Hoechst (blue). Cells exhibit high level of PHEV coat protein at the nuclear periphery, multiple lamellipodia and filopodia, and decreased cortical and cytoplasmic actin.
B) Uninfected HCT-8 control cells. Relative to infected cells, nuclear peripheral signal of PHEV coat protein is absent, appearance of lamellipodia and filopodia is reduced, and bright ubiquitous cortical and cytoplasmic actin is abundant. Kindly provided by Prof. Wenqi He, from Lv et al. 2019).
Citation Spotlight: Arp2/3 regulates RhoA activity and protein levels through cortactin/p190RhoGAP and CCM2/Smurf1, respectively.
Recently, the potential for the actin cytoskeleton (e.g., actin-binding protein complex Arp2/3) to regulate the activity and protein expression of upstream Rho-family GTPases (e.g., RhoA, Rac1, Cdc42) was evaluated. Genetic or pharmacological inactivation of Arp2/3, which controls actin filament branching, reduced contractility and correlated with decreased myosin II and RhoA (but not Rac1 or Cdc42) activation. The GTPase-activating protein p190RhoGAP reduced RhoA activity through increased physical interactions between the two proteins. P190RhoGAP is normally inhibited via direct cortactin binding, but following Arp2/3 inhibition, cortactin dissociated from p190RhoGAP. Surprisingly, RhoA (but not Rac1, Cdc42, or p190RhoGAP) protein levels increased due to reduced RhoA ubiquitination mediated by the adaptor protein CCM2 (cerebral cavernous malformation 2) and the E3 ubiquitin ligase Smurf1 and subsequent proteasomal degradation. Furthermore, Arp2/3 inhibition and concomitant reduction in active RhoA/increase in total RhoA resulted in defects in cytokinesis which were rescued with a cell-permeable Rho activator. Cytoskeleton’s RhoA, Rac1, and Cdc42 antibodies; rhotekin-RBD and PAK-PBD beads; Acti-stain phalloidins; and Rho activator II (Cat.# ARH04, ARC03, ACD03, RT02, PAK02, PHDG1, PHDR1, and CN03, respectively) were essential in identifying a specific feedback loop between Arp2/3 and RhoA that reveals a previously unknown actin-based regulatory role in RhoA GTPase-mediated signal transduction.
Link to citation: Huang Y. et al. 2019. Arp2/3-branched actin maintains an active pool of GTP-RhoA and controls RhoA abundance. Cells. 8, 1264.
Products used in this citation:
Anti-Cdc42: mouse Mab - (Cat. # ACD03)
Anti-RhoA: mouse Mab - (Cat. # ARH04) - Replaced by ARH05 - available here
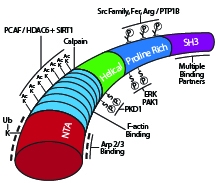
Schematic diagram of cortactin’s primary structure with key post-translational modifications and binding domains highlighted.
Citation Spotlight: Xenopus Egg Cytoplasm Self-assembles into Spatially Organized, Cell-like Compartments De Novo
Recently, Xenopus laevis eggs were used to study if homogenized egg cytoplasm can support spontaneous cellular re-assembly with restored macromolecular structures and functionality. Bright-field and fluorescent microscopy demonstrated that the cytoplasm of eggs arrested at interphase and homogenized can undergo self-assembly into sheets of 300-400 micron long, cell-like compartments in ~30 minutes. Successful self-assembly required formation of microtubules (MTs), but not F-actin, minus-end-directed cytoplasmic dynein motor activity, and adenosine triphosphate. MTs in the cell-like compartments appeared similar to the MTs of intact Xenopus embryos. Neither nuclei (chromatin) nor centrosomes were needed for self-assembly initiation. Genomic input was minimized, if not fully precluded, by inhibition of protein translation with cycloheximide. Introduction of demembranated sperm nuclei as a source of centrosomes and DNA enabled the cell-like compartments to undergo multiple cycles of mitosis if cycloheximide was excluded. Cytoskeleton’s SiR-actin and SiR-tubulin live cell imaging probes, along with green and far-red fluorescently-labeled tubulins (Cat.# CY-SC001, CY-SC002, TL488M, TL670M, respectively), revealed the dynamic re-organization, subcellular localization, and functional roles of MTs and F-actin in the formation of the cell-like compartments. The study confirms that cytoplasmic components of Xenopus egg extracts provide the necessary macromolecular complexes, spatial organization, and cell cycle function to initiate self-organization.
Link to citation: Cheng X. and Ferrell Jr. J.E. 2019. Spontaneous emergence of cell-like organization in Xenopus egg extracts. Science. 366, 631-637.
Products used in this citation:
HeLa cell expressing mcherry-H2B (red) stained with SiR-tubulin (green). Data collected by confocal imaging. Courtesy of Daniel Gerlich and Claudia Blaukopf, Institute of Molecular Biotechnology, Vienna.
Citation Spotlight: Transient Calcium Waves, Actin Dynamics, and Wound Healing
Recently, Lee et al. used a variety of in vitro and ex vivo experimental techniques, as well as custom MATLAB analyses, to investigate transient calcium waves as a means of cell-to-cell communication to coordinate the collective migration of human corneal limbal epithelial (HCLE) cells in wound healing. Following injury, cells release the nucleotide ATP which binds P2X7 and P2Y2 purinergic receptors, triggering release of calcium and activation of associated mechanotransduction signaling pathways. The authors report that the injury-induced, sustained calcium mobilizations are mediated by pannexin channels, allowing epithelial cells to communicate and coordinate changes in the cells’ actin-based morphology to enable migration to the injury site. Calcium mobilizations are correlated with these morphological changes and subsequent increased motility, both of which require dynamic re-organization of the actin cytoskeleton. Cytoskeleton’s SiR-actin live cell imaging probe (Cat.# CY-SC001) was essential in this study as it provided a sensitive and specific method to perform short and long-term live cell confocal imaging of morphological changes in the actin cytoskeleton of HCLE cells during pharmacological manipulation of the pannexin-supported calcium mobilizations to study how different populations of HCLE cells respond to injury and participate in wound healing.
Products used in this citation:
HeLa cells expressing H2B-mCherry (Blue) stained with SiR-actin (red). Image taken by confocal microscopy. Courtesy of Daniel Gerlich and Claudia Blaukopf, Institute of Molecular Biotechnology, Vienna.
Citation Spotlight: Polyglutamylation Regulates Motility of Neuron-specific KIF1A Kinesin
Recently, Lessard et al. investigated how polyglutamylation of tubulin’s C-terminal tails (CTTs) regulates processivity of the neuron-specific kinesin motor KIF1A. KIF1A transports neuronal cargo anterogradely along axonal microtubules (MTs) over long distances (~3 microns). Prior studies implicated interactions between the K-loop of KIF1A and polyglutamylated CTTs in the regulation of KIF1A motility. The K-loop is a lysine-rich surface loop that attaches the motor to MTs via interactions with glutamate-enriched tubulin CTTs. Single-molecule total internal reflection fluorescence microscopy was utilized to better understand how polyglutamylation regulates KIF1A motility. Dimeric KIF1A exhibited novel, stochastic pausing behavior during motility on paclitaxel-stabilized MTs. Such pauses enabled KIF1A to combine multiple sequential runs into a super-processive run length. Furthermore, the K-loop/CTT interaction positively regulated KIF1A’s landing rates onto MTs and pause frequency and duration. Moreover, CTT polyglutamylation regulated this interaction and KIF1A’s subsequent motile behavior. Cytoskeleton’s 99% pure rhodamine-labeled porcine brain tubulin (Cat.# TL590M) was essential as the motor substrate for TIRF microscopic analyses of how K-loop/CTT interactions regulate KIF1A’s processivity. These results provide novel mechanistic insights into KIF1A-mediated axonal transport and the regulatory role of tubulin CTT polyglutamylation, which may further clarify how dysfunctional MT-based, kinesin-mediated axonal transport contributes to neurodegeneration.
Products used in this citation:
KIF1A kinesin motor proteins transport cargo anterogradely along microtubules in the axons of neurons.
Citation Spotlight: Tctex-1, Novel Binding Partner of KIM-1, and Phagocytosis of Apoptotic Cells
Ismail et al. characterized Tctex-1, a novel binding partner of kidney injury molecule-1 (KIM-1), and described the role both proteins have in efferocytosis, the phagocytosis of apoptotic cells. Efferocytosis is an important function in tissue repair, reducing inflammation and abnormal autoimmune responses. KIM-1 is a phosphatidylserine (PS) receptor and PS is a well-known identifier of apoptotic cells. The physical and functional interactions between Tctex-1 and KIM-1 were examined in proximal tubular epithelial cells (PTECs) which can act as amateur phagocytes. A previously unknown role for Tctex-1 in KIM-1-mediated efferocytosis in PTECs was described. Efferocytosis requires re-organization of the actin and microtubule networks, prompting the authors to examine actin cytoskeletal dynamics and the potential role of RhoA and Rac1 in efferocytosis mediated by Tctex-1/KIM-1. Notably, neither RhoA nor Rac1 activities were regulated by Tctex-1 during KIM-1-dependent efferocytosis in PTECs. These data suggested the unexpected hypothesis that non-canonical pathways not involving RhoA and Rac1 are utilized by Tctex-1. Cytoskeleton’s rhodamine-phalloidin, RhoA and Rac1 G-LISA activation assay kits, and Total RhoA ELISA (Cat. # PHDR1, BK124, BK128, BK150, respectively) were essential reagents in this study, the first to describe the physical and functional roles of Tctex-1 in KIM-1-dependent efferocytosis in kidney cells.
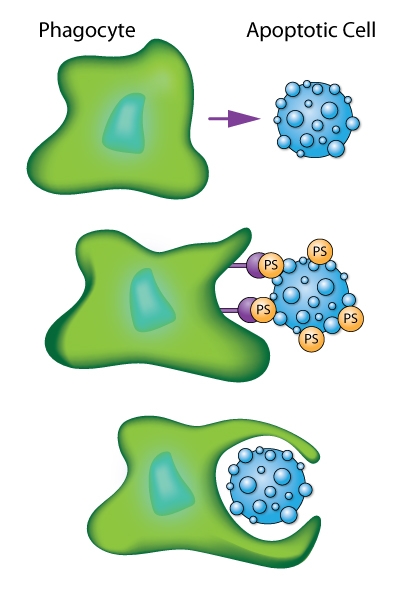
Schematic of phagocytic cell engulfing an apoptotic cell as mediated by recognition of phosphatidylserine (PS) signals.
Citation Spotlight: Misshapen Kinase Alters Actin Dynamics in the Regulation of Ring Canal Size and Stability
Kline et al. recently studied regulation of intercellular bridges in Drosophila egg chambers. Known as ring canals, the bridges transfer cytoplasmic materials between neighboring cells; in this case, from nurse cells to the oocyte over the course of oogenesis. Ring canals are essential for maintaining an organism’s fertility. Ring canal size and stability rely upon dynamic re-organization of F-actin. Here, the Ste20 family kinase misshapen (msn) has a novel role in regulating ring canal size and stability with changes in msn expression levels or localization altering the F-actin cytoskeleton. Phosphotyrosine (pTyr) signal in ring canals strongly overlaps with actin in control egg chambers. pTyr signal was used as a read-out to measure msn localization and activity in control vs msn-RNAi-treated egg chambers. In the latter, pTyr localized to nurse cell membranes and most ring canals. However, pTyr fluorescence in ring canals was variable, even within the same egg chamber, and did not overlap with actin signal. Hence, msn is not necessary for recruiting actin to the ring canal, but is essential for maintaining a ring canal’s actin-based structure. Cytoskeleton’s anti-phosphotyrosine antibody (Cat.# APY03; clone 11G2) was essential in the subcellular localization of pTyr signal in control and msn-depleted ring canals.
Products used in this citation:
Phosphotyrosine Antibody Mouse Monoclonal 27B10 (Cat. # APY03)
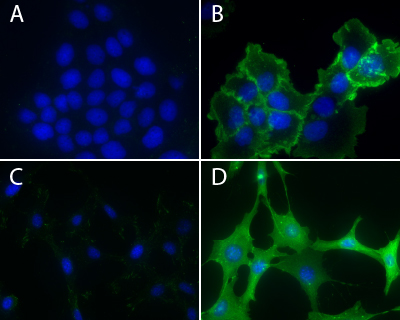
Immunofluorescence using Phosphotyrosine Antibody: Human epidermoid carcinoma A431 cells, untreated (3A) or treated (3B) with EGF (100 ng/ml for 3 minutes), and NIH3T3, untreated (3C) or treated (3D) with H2O2-activated sodium orthovanadate (100 µM for 10 minutes), were stained as described in the method. Phosphotyrosine and nuclei were visualized in green fluorescence and blue DAPI staining, respectively.
Citation Spotlight: Regulating RhoA Activity by Modulating RhoGDI Alpha Post-Translational Modifications
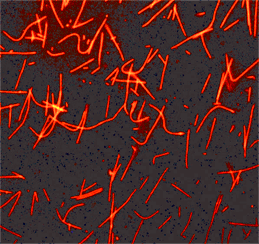
Scientists at Cytoskeleton Inc. recently investigated post-translational modifications (PTMs) that regulate the Rho GTPase family of proteins. Here, the authors characterized the functional regulation of RhoGDIα by SUMOylation 2/3 (SUMO 2/3) and acetyl-lysine PTMs. These studies led to the identification of endogenous SUMO 2/3 and acetylation (Ac) modifications of RhoGDIα. Interestingly, the microtubule stabilizer paclitaxel induced both Ac and SUMO 2/3 of RhoGDIa in a time-dependent fashion. These changes in the PTM state of RhoGDIα altered the protein’s ability to inhibit RhoA activity. Experiments performed with the HDAC inhibitor, TSA, provided evidence of crosstalk between RhoGDIa SUMO 2/3 and Ac in a cell-type dependent fashion and further support the hypothesis that these PTMs may alter RhoGDIa’s function. Cytoskeleton's SUMO 2/3, and Ac Signal-Seeker™ PTM detection kits (Cat. # BK162, and BK163, respectively) were essential reagents in this study, providing a novel toolset for simple and effective investigation of established and novel PTMs for any target protein.
Link to citation:
Products used in this citation:
Signal-Seeker™ Phosphotyrosine Detection Kit (Cat. # BK160)
Signal-Seeker™ Ubiquitination Detection Kit (Cat. # BK161)
Signal-Seeker™ SUMOylation 2/3 Detection Kit (Cat. # BK162)
Signal-Seeker™ Acetyl-Lysine Detection Kit (Cat. # BK163)
BlastR Rapid Lysate Prep Kit (Cat. # BLR01)
Paclitaxel (Taxol) (Cat. # TXD01)
Acti-stain™ 488 phalloidin (Cat. # PHDG1)
RhoA G-LISA Activation Assay Kit (Colorimetric format) 96 assays (Cat. # BK124)
Citation Spotlight: Actin Bundling Protein EFhd2 Mediates LPS-Induced Macrophage Migration
Tu et al. recently characterized the functional relationship between the novel actin bundling protein EFhd2 and actin during lipopolysaccharide (LPS)-induced macrophage migration, an important component of innate immune responses. The authors found that LPS-mediated macrophage migration depends upon EFhd2 and its regulation of actin polymerization and subsequent re-organization of the actin cytoskeleton. EFhd2 and F-actin co-localization in macrophages is essential for LPS’s actions and coincides with a LPS-induced increase in the ratio of F-actin to G-actin in these cells, an effect prevented by knock-out of EFhd2. Under cell-free conditions, EFhd2 increases actin polymerization in a concentration-dependent manner and to a magnitude similar to that induced by the nucleation factors Arp2/3 + VCA domain of the Wiskott-Aldrich syndrome protein (WASP). This suggests that EFhd2’s effects are through a direct regulation of the actin cytoskeleton. Cytoskeleton’s actin polymerization assay kit, G-actin/F-actin in vivo assay kit, Acti-stain 555 phalloidin, Arp2/3 protein complex, and VCA domain of WASP (Cat. # BK003, BK037, PHDH1, RP01P, and VCG03, respectively) were essential reagents in this characterization study. These kits and reagents provided the tools necessary to examine how EFhd2 and its regulation of actin polymerization and cytoskeletal dynamics participate in LPS-induced macrophage motility.
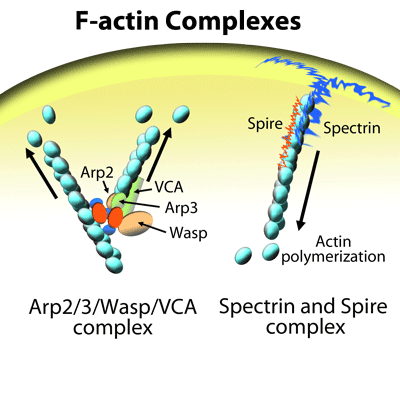
Nucleation of actin filaments (F-actin) mediated by a complex consisting of the actin nucleation factors Arp2, Arp3, and WASP (VCA domain).
Citation Spotlight: Acetylated Microtubule Bundling, MAP Binding, and Functional Regulation of Kinesin-1
Balabanian et al. recently investigated the functional regulation of kinesin-1 motility using intact microtubules (MTs) in the form of either a single filament or bundles that were isolated from living COS-7 cells. Post-translational modifications (PTMs), MT bundling, and binding of microtubule-associated proteins (MAPs) are influential modulators of MT stability and motor protein activity. Here, the authors investigated the effects of the acetylation PTM, MT bundling, and binding of the MAP tau on kinesin-1 motility. Cytoskeleton’s SiR-tubulin live cell imaging probe and paclitaxel (Cat. # CY-SC002 and TXD01, respectively) were essential reagents in this kinesin-1 motility study, providing the tools necessary to examine the MT network in living cells and then confirm that it was maintained upon detergent extraction and isolation, followed by the necessary paclitaxel stabilization. The in vitro paclitaxel-stabilized, isolated MT single filaments and bundles faithfully recapitulated important structural attributes of an intact MT network in living cells. This in vitro model enabled the study of how kinesin-1 is functionally regulated by MT architecture, MAP binding, and PTMs such as acetylation. In sum, this model system provides a greater understanding of the physiological regulation of kinesin motors and kinesin-regulated transport of cargoes along MTs.
Link to citation: Balabanian L. et al. 2017. Acetylated microtubules are preferentially bundled leading to enhanced kinesin-1 motility. Biophys. J. 113, 1551-1560.
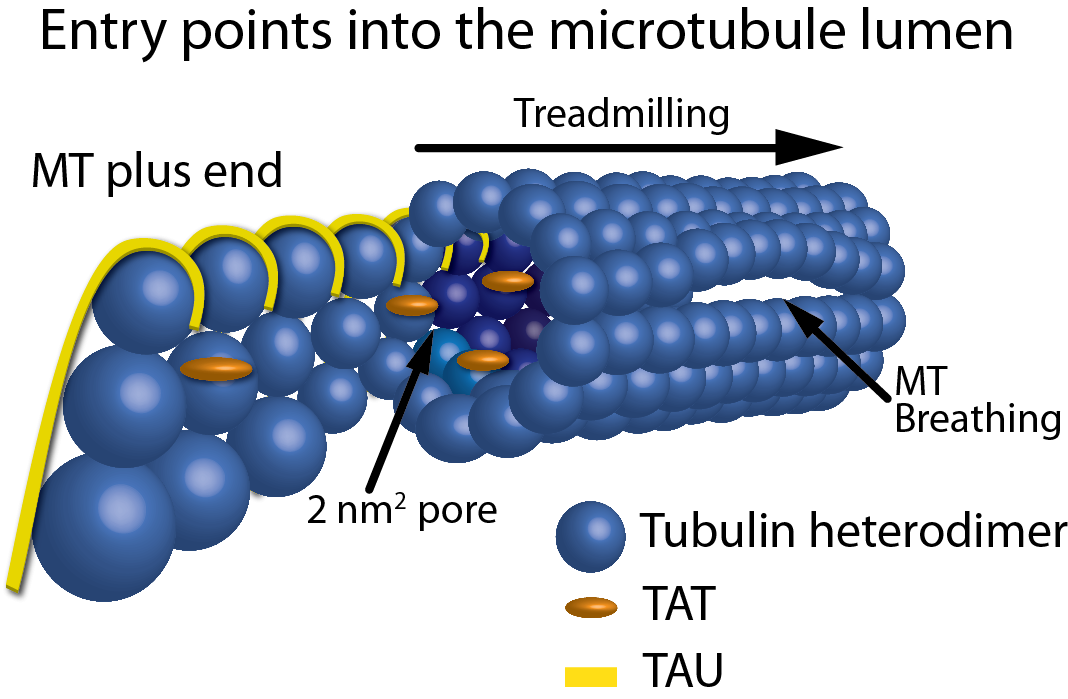
Figure 1: Schematic representation of the entry points into the MT lumen. Showing, from left to right, a frayed/growing MT plus end capturing TAT and tau molecules, treadmilling, 2 nm2 pores, a 200 nm2 open MT plus end, and a breathing MT lattice.
Citation Spotlight: Anti-cancer Therapeutic Buparlisib: Mitosis or PI3K Inhibitor?
Bohnacker et al. recently investigated the primary site of action of the anti-cancer therapeutic BKM120 (a.k.a., Buparlisib), a clinically-advanced phosphoinositide 3-kinase (PI3K) inhibitor. Although PI3K inhibition is considered the primary mechanism of action, some studies report that BKM120 exerts potent off-target effects on tubulin, which raises questions about its substrate. Here, the authors aimed to decipher BKM120’s molecular interactions with tubulin and PI3K to identify its anti-tumorigenic site of action. The authors reported that BKM120’s anti-cancer activity is through mitotic arrest via microtubule destabilization, rather than PI3K inhibition. Chemical derivatives of BK120 were synthesized and examined to separate targeting of PI3K versus tubulin in pursuit of a more potent and selective PI3K inhibitor. The BKM120-derived molecule, PQR309, strongly inhibited PI3K with no detectable effect on tubulin polymerization and/or microtubule stability. Cytoskeleton’s 99% pure porcine brain tubulin, tubulin polymerization assay kit, biotinylated porcine brain tubulin, and TRITC rhodamine-labeled tubulin (Cat.# T240, BK006P, T333P, and TL590M, respectively) were essential reagents in this study, providing the tools necessary to examine how BKM120 and BKM120-derived molecules affected tubulin polymerization and microtubule dynamics as assessed by in vitro assembly and microtubule plus-end tracking assays, with the goal of designing novel, specific, and potent PI3K inhibitors.
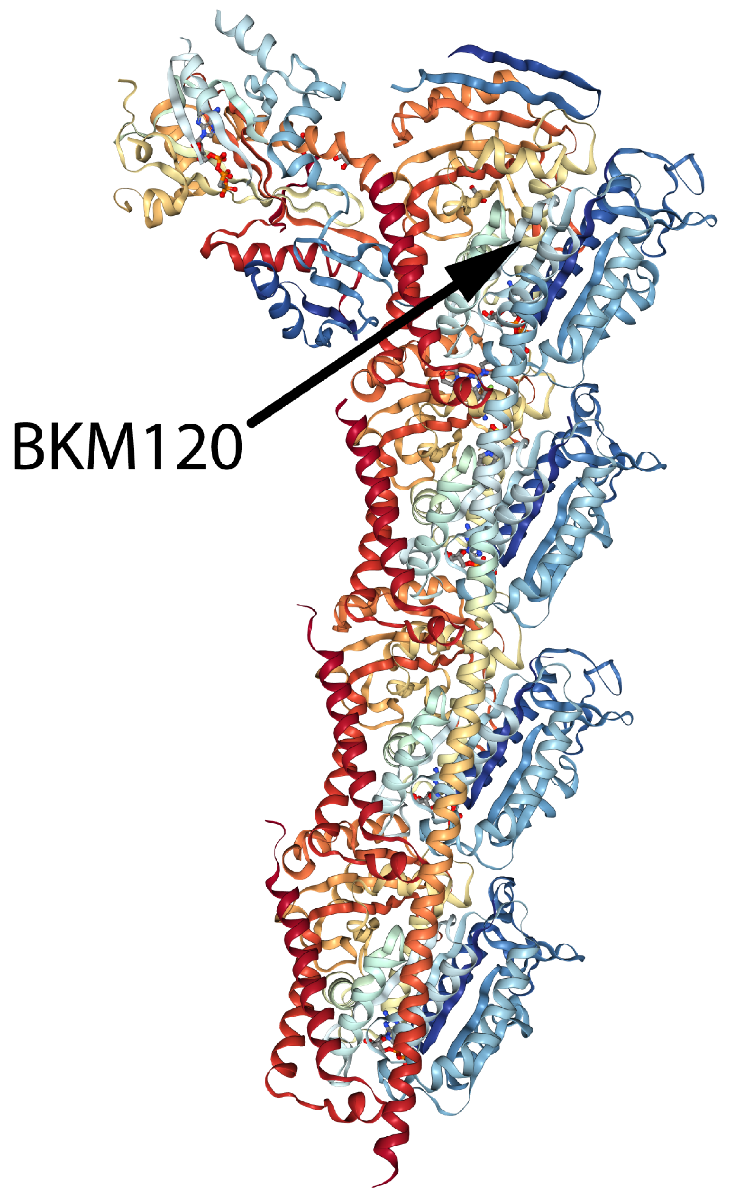
Alpha/beta tubulin heterodimer in complex with tubulin tyrosine ligase, stathmin-4, and the small molecule compound BKM120 (Buparlisib).
Citation Spotlight: Rho-Family GTPase Activity: Key Components of Anti-Inflammatory and Neuroprotective Pathway
Recently, Varadaraj et al. examined transforming growth factor ß (TGF-ß) regulation of the extracellular matrix (ECM) protein fibronectin (FN). Soluble FN dimers polymerize to form insoluble, matrix-associated FN polymers in a process known as fibrillogenesis. The resulting FN fibril network is a scaffold for cell migration, repair, and adhesion mediated by binding to a5ß1 integrin receptors. TGF-ß stimulates ECM remodeling and cell migration through the induction of FN fibrillogenesis, which is necessary for TGF-ß’s effects. Here, the authors found that FN’s role in TGF-ß-mediated ECM remodeling and cell migration can occur via increased FN trafficking, i.e., recycling between the plasma membrane and cytosol. In response to TGF-β, cell surface FN is endocytosed and undergoes Rab11-mediated recycling and subsequent incorporation into fibrils, a process dependent on an interaction between the cytoplasmic domain of the type II TGF-β receptor (TβRII) and a5ß1 integrin. Cytoskeleton’s rhodamine-labeled and biotinylated fibronectin (Cat. # FNR01 and FNR03, respectively) were essential reagents in this interesting study, providing the tools necessary to discover that TGF-ß induces FN trafficking/recycling, a novel process that offers a rapid pathway by which FN can regulate cell migration, wound repair, and fibrosis.
FNR01 image overlay with phase contrast background. Fluorescent fibronectin (Cat. # FNR01) treated MCF10A cells (image kindly provided by A. Varadaraj and M. Karthikenyan, Univ. S.Carolina, Columbia, SC).
Citation Spotlight: Characterization of Novel 3D In Vitro Model of Cancer Cell Invasion
Recently, Guzman et al. characterized a novel 3D in vitro model of multicellular cancer cell invasion that offers adjustable physiological and biochemical parameters. Shortcomings of current models are poor microscopic imaging dynamics and inability to study transmigration of cancer cells across the basement membrane (BM) and then invasion of an extracellular matrix (ECM), as happens in vivo. The BM is a type of ECM that surrounds a tumor, formed in a multi-step process initiated by cancer cells binding laminin at the cell surface to form a scaffold upon which type IV collagen polymers form. This new model offers improved imaging dynamics while also recapitulating in vivo tumor cytoarchitecture with an intact, degradable, cell-assembled, sheet-like BM layer embedded in a collagen I-enriched ECM. Cytoskeleton’s HiLyte488-conjugated laminin (Cat. # LMN02) was used to confirm that in vitro BM layer formation paralleled the in vivo process; that is, the laminin scaffold was dispersed across the surface of the multicellular tumor spheroids in a thin, patchy layer, serving as the foundation of the BM layer. This innovative 3D cancer cell invasion model offers researchers the ability to both tease apart molecular mechanisms underlying invasion and test new therapeutics in a physiologically- and biochemically-relevant setting.
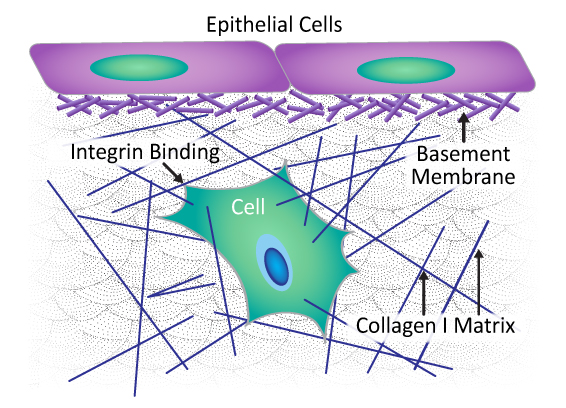
Composition and architecture of extracellular matrix (ECM). Basement membrane is a type of ECM composed of laminin and collagen IV fibers embedded within a collagen I-enriched ECM.
Citation Spotlight: Rho-Family GTPase Activity: Key Components of Anti-Inflammatory and Neuroprotective Pathway
Recently, Rom et al. examined molecular pathways involved in leukocyte-mediated neuroinflammation given its causative role in neuronal dysfunction associated with brain injuries and diseases. Neuroinflammation involves a compromised blood-brain barrier (BBB) as leukocytes need to engage brain endothelial cells. To do so, leukocytes utilize integrin adhesion receptors for rolling, arrest, adhesion, and transendothelial migration (TEM), processes requiring Rho-family GTPase-mediated rearrangement of the actin cytoskeleton. Here, the activation of VLA-4 and LFA-1 leukocyte integrins following inhibition of PARP (poly(ADP-ribose) polymerase 1) activity in leukocytes was studied with the goal of preventing BBB breakdown. Using primary human brain microvascular endothelial cells to model the BBB, PARP inhibitors reduced leukocyte adhesion and TEM, concomitant with decreased activation of the two integrins and RhoA and Rac1 GTPases, as well as a reduced F-/G-actin ratio. Cytoskeleton’s RhoA and Rac1 G-LISA activation assays (Cat.# BK124 and BK128, respectively), Acti-stain 488 phalloidin (Cat.# PHDG1), and cell-permeable Rho inhibitor (Cat.# CT04) and Rho/Rac/Cdc42 activator (Cat.# CN04) were essential reagents, allowing for sensitive and reliable quantification of RhoA and Rac1 activation under control and experimental conditions while also measuring dynamic actin cytoskeletal changes. These results suggest novel therapies for protecting BBB integrity following brain disease and injury.
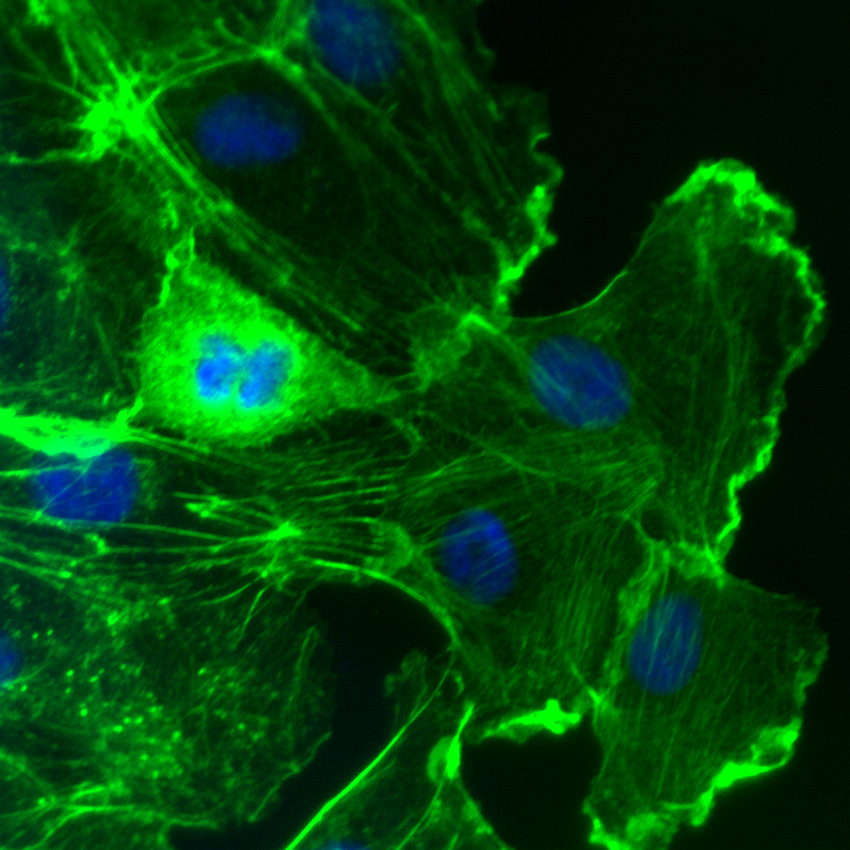
Rac activation in Swiss 3T3 fibroblasts. F-actin is visualized with fluorescent green phalloidin staining (Cat.# PHDG1). DAPI is the blue nuclear stain. Phalloidin staining shows F-actin-rich lamellipodia. Cells were activated with Cat.# CN04.