SPY620-DNA is a bright red & non toxic live cell nuclear stain based on our SPY™ dyes series. Its optimized structure allows quick labeling of DNA in live & fixed cells with high specificity and very low background. SPY620-DNA stains the nuclei of live or fixed cells without the need for genetic manipulation or overexpression of fluorescent proteins. Its emission in the red minimizes phototoxicity and sample autofluorescence. SPY620-DNA enables multicolor imaging with SPY505, SPY555, SPY650, SPY700, SiR and GFP. SPY620-DNA can be imaged with e.g. 610/20 nm and 650/40 nm excitation and emission filters respectively. It can be used for widefield, confocal, SIM or STED imaging in living or fixed cells and tissue. Contains 1 vial of SPY620-DNA (lyophilized).
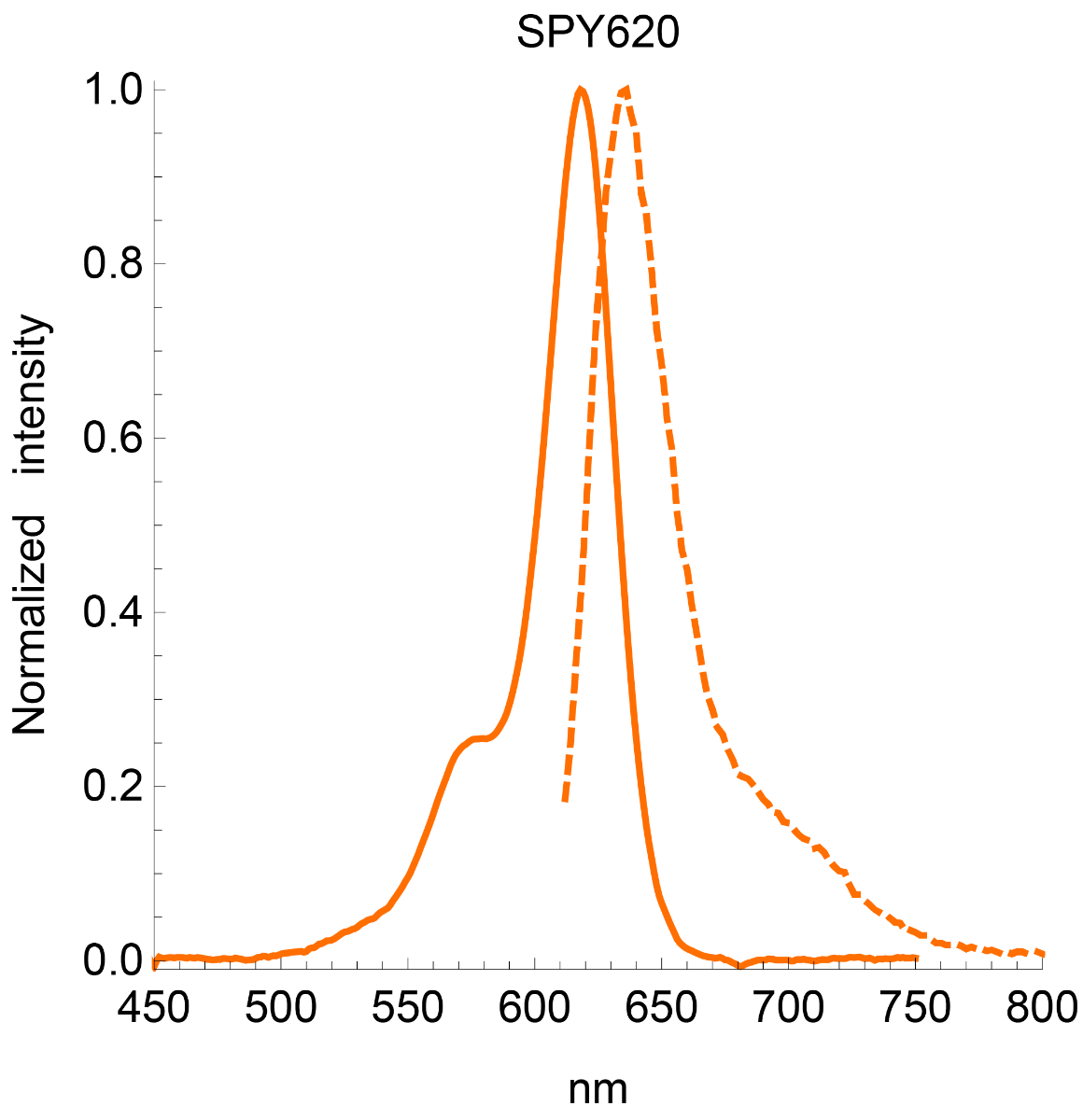
Absorbance maximum λabs | 619 nm |
Fluorescence maximum λfl | 636 nm |
Works on fixed cells? | yes, PFA and methanol |
Probe quantity | 100 stainings* |
Fluorescence lifetime | 2.9 ns |
STED depletion wavelength | 775 nm |
Shipping | room temperature |
Storage | -20°C |
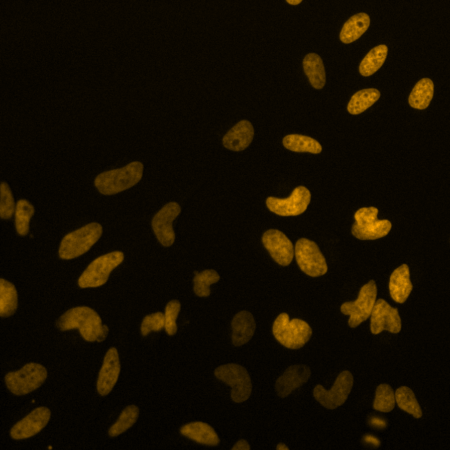
HeLa cells stained with SPY620-DNA. Image courtesy of Spirochrome
For product Datasheets and MSDSs please click on the PDF links below.
Spirochrome Technical Tips and Ex/Em spectra in graphical form (PDF)
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
Q1. What is STED microscopy and how does it work?
A1. STED microscopy stands for Stimulated Emission Depletion microscopy. It is one type of super resolution microscopy which allows the capture of images with a higher resolution than conventional light microscopy which is constrained by diffraction of light. STED uses 2 laser pulses, one is the excitation pulse which excites the fluorophore, causing it to fluoresce. The second pulse, referred to as the STED pulse, de-excites the fluorophore via stimulated emission in an area surrounding a central focal spot that is not de-excited and thus continues to fluoresce. This is accomplished by focusing the STED pulse into a ring shape, a so-called donut, where the center focal spot is devoid of the STED laser pulse, conferring high resolution to the fluorescent area (Fig. 1; see Ref. 1 for more details on STED microscopy).
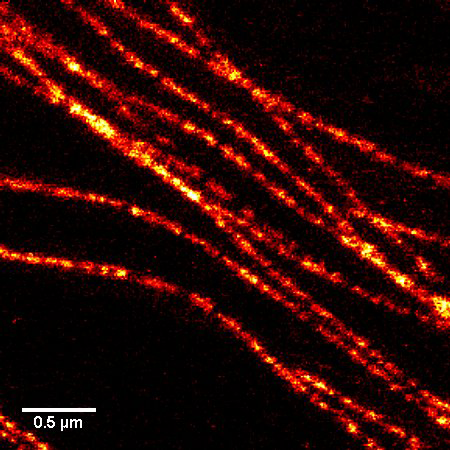
Figure 1. STED microscopic image of microtubules labeled with SiR-tubulin in human primary dermal fibroblasts.
Q2. Why is the SPY actin (or tubulin/DNA) probe good for STED microscopy?
A2. STED microscopy offers the ability to study cellular details on a nanometermolar scale in vivo. To take advantage of this super resolution microscopy, one must be able to select with high specificity the area to be examined using fluorescent probes. In addition, the fluorescent probes must be bright, photostable, exhibit no or little phototoxicity, be excited and emit in the far red spectrum. In addition, if the probe is to be used for live cell imaging (thus avoiding fixation artifacts that occur when cells are fixed), high cell permeability is necessary. The SPY actin and tubulin probes fulfill all of these requirements. In short, the combination of STED and SiR probes allows for unparalleled fluorescent visualization of subcellular actin and tubulin/microtubule structures and their physical characterization in living cells, (see Fig. 2 and Ref. 2).
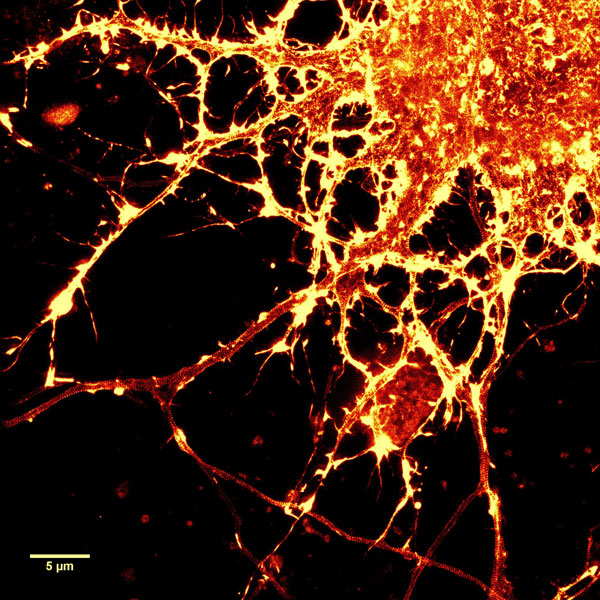
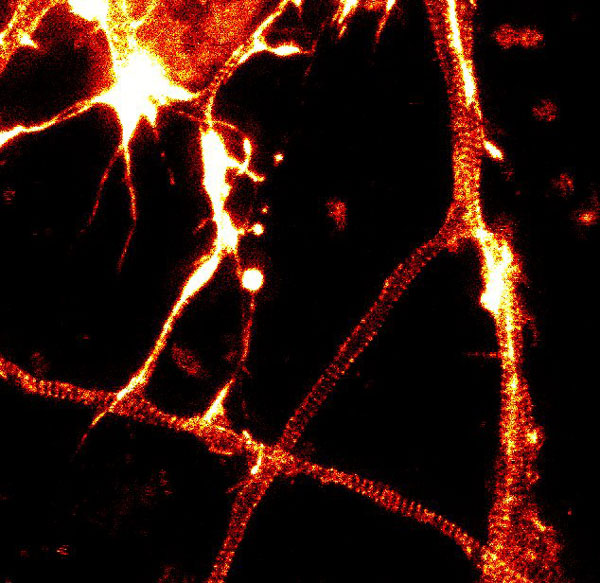
Figure 2. STED images of cultured rat hippocampal neurons stained with SiR-actin. Bottom image is a close-up view of part of the top image to clearly visualize actin rings (stripes) with 180 nm periodicity. Courtesy Of Elisa D'Este, MPI Biophysical Chemistry, Göttingen.
Q3: Are the SPY probes stable at room temperature?
A3: Yes, the probes are stable at room temperature for a few days. However, it strongly depends on the probe and the solvent. Thus, it is recommended to store all of the probes or solutions at –20°C.
Q4: Are SPY-actin, SPY-DNA and SPY-tubulin toxic to cells?
A4: Yes, above a certain threshold both probes show some effect on cell proliferation and altered actin or microtubule dynamics. However, the probes are orders of magnitude less toxic than their parent drug. In HeLa cells, neither actin nor microtubule dynamics were altered at concentrations below 100 nM. At this concentration, SPY probes efficiently label microtubules and F-actin, allowing for the capture of high signal to noise images.
References
1. Hell S.W. and Wichmann J. 1994. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780-782.
2. D’Este E. et al. 2015. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 10, 1246-1251.
3. Lukinavicius G. et al. 2013. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 5, 132-139.
4. Lukinavicius G. et al. 2014. Fluorogenic probes for live-cell imaging of the cytoskeleton.Nature Methods. 11, 731-733.
