Tubulin K40 Acetylation in Neurons: Novel Functions, Regulators, and Modifications
Introduction
Tubulin α/β subunits are the key building blocks for microtubule (MT) formation, and the ability of MTs to dynamically switch between growth and shrinkage is a hallmark feature of these cytoskeletal structures. Microtubules have many cellular functions including facilitating cell shape, cell movement, cell division, control of organelle transport, and many others. Therefore, proper cell function is dictated by strict regulation of MT dynamics, which is controlled by several mechanisms including microtubule associated proteins (MAPs), signaling pathways, and post-translational modifications (PTMs).
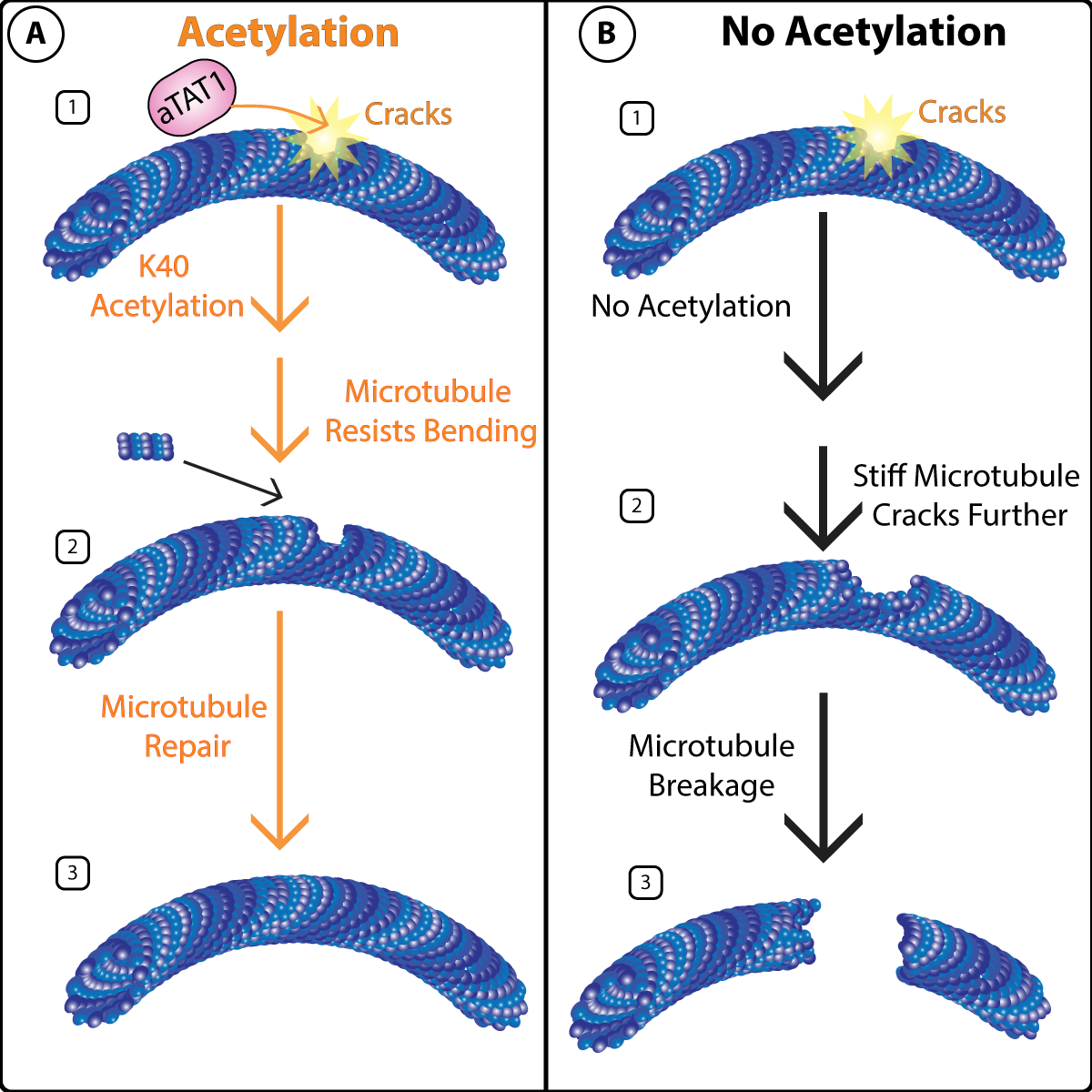
In recent years there has been an abundance of evidence that tubulin is highly post-translationally modified, and these PTMs such as de-tyrosination, acetylation and polyglutamylation are critical for proper cell function(1). What’s more, dysfunction of these PTMs have been linked to human disease(2). Tubulin PTMs are highly studied in neurons because of the abundance of tubulin PTMs observed in these cells, as well as their link to neuronal disease. In particular, several studies have linked dysfunctional tubulin acetylation to neuronal diseases such as Charcot-Marie-Tooth disease and Alzheimer’s disease (AD)(3, 4). Acetylation of tubulin occurs primarily on the lysine at position 40 (K40) on the α-tubulins of assembled MTs(reviewed in (5)). The predominant alpha-tubulin acetyltransferase is αTAT1. Deacetylation of acetylated tubulin is mediated by histone deacetylase 6 (HDAC6) or SIRT2. Acetylation at K40 has been shown to stabilize MTs (see Figure1), which promotes a long-lived phenotype in-part through modulating inter-protofilament interactions, stabilizing the MT lattice, and allowing for self-repair(reviewed in (5)). Here we discuss newly identified functions of key regulators, novel binding partners, and competing PTMs that interact with this critical tubulin PTM and their effect on neurons.
Non-Enzymatic αTAT Activity Influences MT Dynamics and Bouton Growth in Neurons
αTAT1 acetylates tubulin at K40 on the luminal surface of MTs, which results in a stable and long-lived form of MTs(6). Early studies by Kalebic et al. suggested that αTAT1 may also have non-enzymatic functions that alter MT dynamics(7). This dual functionality is intriguing because in neuronal cells there is a need for both long-lived MTs to stabilize axonal outgrowths, as well as highly dynamic MTs in synaptic boutons. Coombes et al. recently investigated whether αTAT1 was playing a role in MT dynamics in these neuronal structures, and discovered that indeed the non-enzymatic function of αTAT1 had profound effects on MT dynamics(8). Using a Drosophila neuromuscular junction (NMJ) model they showed that depletion of dαTAT resulted in much more dynamic tubulin activity as measured by a significant increase in ectopic bouton formation. Rescue with either wild-type or acetyltransferase-inactive dαTAT had the same effect on bouton growth, suggesting that these effects were independent of it enzymatic function. They utilized cell free models to show that non-enzymatic αTAT1 caused catastrophe in dynamic MTs but not long-lived MTs. Collectively, this suggests that αTAT1 enriches for long-lived MTs by two mechanisms, 1. Acetylation at K40 to enhance stability, and through a non-enzymatic mechanism that promotes destabilization of dynamic MTs. It appears that both mechanisms are active and important in proper MT function in neurons.
ID2 is a Novel Binding Partner of MTs and Promotes K40 Acetylation
Sirt2 has been identified as a deacetylase for tubulin that has been shown to be abnormally expressed in some AD models, resulting in MT destabilization and Tau dissociation(9). While the HAT and HDACs that regulate K40 acetylation are known, other interacting proteins that regulate this tubulin PTM has not been exhaustively studied. Yun et al. identified ID2 as a novel α-tubulin binding partner that promotes lysine acetylation on α-tubulin K40, as a mechanism to promote microtubule stabilization in neurons(10). The group utilized extensive molecular biology techniques to prove that the phosphomimetic form of ID2 was important for interacting with α-tubulin, and mechanistically promotes α-tubulin incorporation into MTs. In hippocampal neurons they showed that knockdown of ID2 led to a 20% reduction in K40 acetylation, while overexpression produced robust acetylation at K40 of α-tubulin. Mechanistically, ID2 augments K40 acetylation by competing with Sirt2 for α-tubulin binding based on depletion studies in neurons and supported with proximity ligation assays. These findings correlated in post-mortem brains of human AD patients where ID2 levels were suppressed while Sirt2 levels were enhanced. Finally, the investigators utilized a mouse model of AD and again saw the same inverse results between ID2 and Sirt2, but rescue with ID2 overexpression in this AD mouse model was sufficient to enhance K40 acetylation and promote axon growth. These data identify a novel MT binding partner that is critical for K40 acetylation and proper neuronal function.
K40 of Tubulin is Regulated by Multiple PTMs
While tubulin K40 was first identified as a target for acetylation, recent studies have shown that it is also a target for tri-methylation (K40me3)(11). K40me3 has been shown to be involved in mitosis and cytokinesis; however, its role in post-mitotic cells such as neurons have not been investigated. Xie et al reported that K40me3 sought to address this question and investigated the role of K40me3 in post-mitotic neuronal cells(12). They found that K40me3 is enriched in neuronal progenitor cells and in neurons in the cerebral cortex in embryonic day 14-16. Knockdown of SETD2, the methyltransferase that modifies K40, resulted in impaired neuronal migration that could be rescued with a K40me3 mimetic mutant. K40me3 was preferentially associated with polymerized microtubules in cells, and appeared to promote stabilized MTs in vitro. More so, depletion of K40me3, via SETD2 knockdown, led to reduced MT abundance in neurites and disrupted neuronal polarization. Interestingly, this data suggests that K40me3 and K40 acetylation may have overlapping effects, and their studies showed that, α-TubK40me3 is increased after losing K40 acetylation. However, they noted that k40me3 was sufficient to rescue morphological transition defects, but not other noted changes like axon over branching which has been seen with K40 acetylation depletion in neurons. This suggests that there is still much to learn about the interplay between these PTMS of tubulin, as well as their downstream functions.
Summary
These studies highlight the importance of tubulin K40 acetylation in neurons and the expansive crosstalk and interplay that exists between PTMs and their regulatory proteins. Importantly, they all work in concert to properly fine tune MT function in neurons, and disrupting these small, but important, modifications can lead to deleterious neuronal function and ultimately disastrous neuropathology. New studies continue to appear that shows that tubulin K40 acetylation is not only important in neurons, but also plays a role in diseases like cancer, cardiovascular and others(13, 14). Importantly, the successes of studying K40 acetylation has paved the way towards investigation of other PTMs of tubulin; for example, a recent article identified SUMOylation as a novel PTM of tubulin that can promote MT catastrophe and impede MT polymerization(15). Cytoskeleton Inc., offers reagents for studying the levels of endogenous PTMs such as acetylation and SuMOylation within cells and we hope to aide investigators as they continue to expand our knowledge in this exciting tubulin PTM field.
Coming Soon: Purified Acetylated Tubulin! Email tservice@cytoskeleton.com with inquires.
References
- Roll-Mecak A. The Tubulin Code in Microtubule Dynamics and Information Encoding. Dev Cell. 2020;54(1):7-20.
- Magiera MM, Singh P, Gadadhar S, Janke C. Tubulin Posttranslational Modifications and Emerging Links to Human Disease. Cell. 2018;173(6):1323-7.
- Hempen B, Brion JP. Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55(9):964-72.
- d'Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, et al. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat Med. 2011;17(8):968-74.
- Janke C, Montagnac G. Causes and Consequences of Microtubule Acetylation. Curr Biol. 2017;27(23):R1287-R92.
- Portran D, Schaedel L, Xu Z, Thery M, Nachury MV. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol. 2017;19(4):391-8.
- Kalebic N, Martinez C, Perlas E, Hublitz P, Bilbao-Cortes D, Fiedorczuk K, et al. Tubulin acetyltransferase alphaTAT1 destabilizes microtubules independently of its acetylation activity. Mol Cell Biol. 2013;33(6):1114-23.
- Coombes CE, Saunders HAJ, Mannava AG, Johnson-Schlitz DM, Reid TA, Parmar S, et al. Non-enzymatic Activity of the alpha-Tubulin Acetyltransferase alphaTAT Limits Synaptic Bouton Growth in Neurons. Curr Biol. 2020;30(4):610-23 e5.
- Silva DF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondrial Metabolism Power SIRT2-Dependent Deficient Traffic Causing Alzheimer's-Disease Related Pathology. Mol Neurobiol. 2017;54(6):4021-40.
- Yun T, Ko HR, Jo DK, Park KW, Cho SW, Kim J, et al. Inhibitor of DNA binding 2 (Id2) mediates microtubule polymerization in the brain by regulating alphaK40 acetylation of alpha-tubulin. Cell Death Discov. 2021;7(1):257.
- Park IY, Powell RT, Tripathi DN, Dere R, Ho TH, Blasius TL, et al. Dual Chromatin and Cytoskeletal Remodeling by SETD2. Cell. 2016;166(4):950-62.
- Xie X, Wang S, Li M, Diao L, Pan X, Chen J, et al. alpha-TubK40me3 is required for neuronal polarization and migration by promoting microtubule formation. Nat Commun. 2021;12(1):4113.
- Ge LP, Jin X, Yang YS, Liu XY, Shao ZM, Di GH, et al. Tektin4 loss promotes triple-negative breast cancer metastasis through HDAC6-mediated tubulin deacetylation and increases sensitivity to HDAC6 inhibitor. Oncogene. 2021;40(12):2323-34.
- Ouyang C, Li J, Zheng X, Mu J, Torres G, Wang Q, et al. Deletion of Ulk1 inhibits neointima formation by enhancing KAT2A/GCN5-mediated acetylation of TUBA/alpha-tubulin in vivo. Autophagy. 2021:1-18.
- Feng W, Liu R, Xie X, Diao L, Gao N, Cheng J, et al. SUMOylation of alpha-tubulin is a novel modification regulating microtubule dynamics. J Mol Cell Biol. 2021;13(2):91-103.
Related Products
Purified Tubulins and MAPS
Tubulin protein (97% pure): porcine brain (Cat. # HTS03)
Tubulin Kits
Tubulin polymerization HTS assay using >97% pure tubulin, OD based - Porcine (Cat. # BK004P)
Tubulin polymerization assay using >99% pure tubulin, OD based - Porcine (Cat. # BK006P)
Tubulin polymerization assay using >99% pure tubulin, fluorescence based (Cat. # BK011P)
Microtubule Binding Protein Spin-Down Assay Biochem Kit (Cat. # BK029)
Microtubule/Tubulin In Vivo Assay Biochem Kit (Cat. # BK038)
Signal Seeker™ PTM Detection Kits
New Signal-Seeker™ Acetyl-Lysine Detection Kit (30 assay) (Cat. # BK163)
New Signal-Seeker™ Acetyl-Lysine Detection Kit (10 assay) (Cat. # BK163-S)
Signal-Seeker™ Phosphotyrosine Detection Kit (30 assay) (Cat. # BK160)
Signal-Seeker™ Phosphotyrosine Detection Kit (10 assay) (Cat. # BK160-S)
Signal-Seeker™ Ubiquitination Detection Kit (30 assay) (Cat. # BK161)
Signal-Seeker™ Ubiquitination Detection Kit (10 assay) (Cat. # BK161-S)
Signal-Seeker™ SUMOylation 2/3 Detection Kit (30 assay) (Cat. # BK162)
Signal-Seeker™ SUMOylation 2/3 Detection Kit (10 assay) (Cat. # BK162-S)
Signal-Seeker™ SUMOylation 1 Detection Kit (30 Assay) (Cat. # BK165)
Signal-Seeker™ SUMOylation 1 Detection Kit (10 Assay) (Cat. # BK165-S)