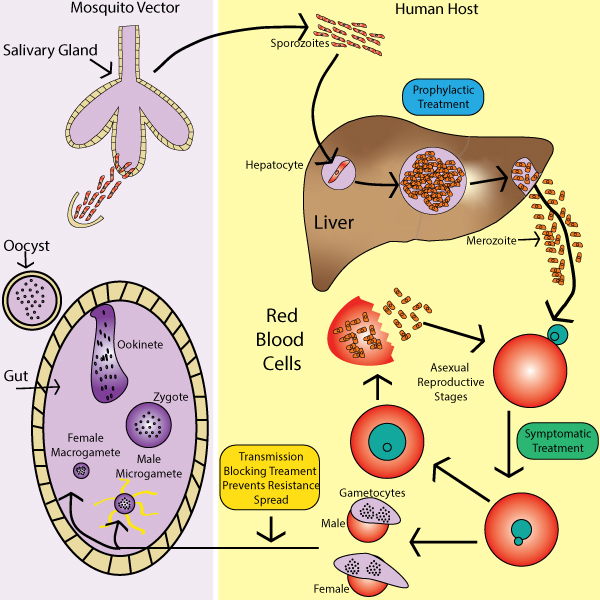
Malaria influences the cytoskeleton during it’s lifecycle
Consequences of Malaria
Malaria has an annual death toll exceeding 400,000 lives and an estimated 229 million cases as recorded worldwide in 2019(1). Africa is the hardest-hit region, as it accounts for >90% of all malaria cases and deaths(2). Children under the age of 5 years are the most vulnerable group to malaria, where approximately 1200 African children die each day(3,4). The transmission of malaria is caused by the protozoal Plasmodium parasite through the bites of female mosquitoes from the Anopheles genus(5). P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi are the five Plasmodium spp. that cause malaria in humans. P. falciparum is the predominant parasitic species that accounts for nearly all the malaria cases in sub-Saharan Africa, while P. vivax is the leading cause of malaria in South America and Southeast Asia(6).
Life Cycle of Plasmodium spp.
To perpetuate the malaria parasitic life cycle, both a human host and a female Anopheles mosquito are required. The malaria infection is initiated during the blood meal, where the Plasmodium sporozoites are injected into the skin of the human host from the Anopheles mosquito (Figure 1). The sporozoites enter the bloodstream and make their way to the liver, where they infect the hepatocytes(reviewed in 3). Each sporozoite in the hepatocyte will generate thousands of merozoites to be released into the bloodstream and repeat the process of infection in erythrocytes. While merozoites repeatedly infect the erythrocytes, some of the merozoites in the red blood cells produce male or female gametocytes. These gametocytes will eventually be ingested by another female Anopheles mosquito after feeding on the infected human host. These gametocytes will mature into ookinetes, which will infect the cells in the mosquito midgut wall. Oocysts are formed on the exterior midgut wall and release sporozoites that travel to the mosquito salivary glands and allows the malaria parasitic infection to continue into a new host.
The life cycle of malaria involves complex rearrangements of the cytoskeletal elements such as microtubules (MT), actin, myosin, and intermediate filaments for each stage of morphogenesis and cell division. Even Sporozoite, ookinete, and merozoite from different Plasmodium spp. can vary in size, morphology, and the number of MTs they can have(7,8). Recent advancements in both microscopy and live-cell imaging probes have been developed for investigating these complex cytoskeletal elements(9). Several recent studies highlighted below, have used Spirochrome’s live imaging probes to stain microtubules (SiR-Tubulin) or F-actin (SiR-actin) of malaria parasites at different invasive stages.
Modulating Microtubules in Plasmodium Impairs Sporozoites
The Plasmodium spp contains three tubulin isotypes of α-tubulin I, α-tubulin II, and ß-tubulin(10). These isotypes are a subset of cytosolic subpellicular MTs in Plasmodium spp. that are found in sporozoites, ookinetes, gametocytes, and merozoites(7, 10). Recently, Spreng B et al. used SiR-tubulin to stain MTs for their gene deletion and replacement of α-tubulin I in P. berghei (rodent malaria species) to test the importance of MT structure in sporozoite infectivity. Modifying the regulatory units of α-tubulin I in a parasite cell line was shown to reduce the number of subpellicular MTs and resulted in deformed sporozoites. Replacement of α-tubulin I with α-tubulin II lead to Sporozites with shorter MTs and reduced the number of subpellicular MTs from 16 to 9 or 11 based on the α-tubulin II parasite line used. Deletion of α-tubulin I did not impair genome replication or nuclear division but was found to prevent sporozoite budding. Additionally, only parasite lines with 10 or more subpellicular MTs were infectious to mice, while sporozoites with shortened MTs had reduced infectivity. These findings show the importance of tubulin regulation in parasitic infection.
Live-cell Imaging of Microgametogenesis in Plasmodium Falciparum
A recent development for live microgametogenesis imaging was performed by Yahiya S et al., where combining live-cell three-dimensional imaging through time allowed for the observation of microgametogenesis from early to late development(11). Previous work in this field was restricted to imaging of fixed samples. The use of SiR-tubulin was found to be specific and photostable for live-cell fluorescence imaging of microgametogenesis. The researchers were able to take labeled gametocytes and add them to ookinete-containing media, where SiR-tubulin helped the researchers to identify different microgametogenesis events. In addition to labeling tubulin, labeling of the host erythrocyte membrane and P. falciparum DNA allowed the researchers to observe the entire process of microgametogenesis in real-time over several hours.
Plasmodium invasion of the Mosquito Midgut
SiR-Actin was used for live-cell imaging of ookinetes traversing midgut epithelial cells(12). Previous work to observe live imaging of ookinete invasion of midguts involved differential interference contrast microscopy and an in vitro system where cultured ookinete was added to mosquito midgut cells(13) and the development of a transgenic P. beghei cell line expressing GFP reporter protein(14). These studies were limited in the amount of time the cells could be imaged, difficulty imaging along the “Z’ axis, and the inability to capture certain infection events. Trisnadi N and Barillas-Mury C developed a new strategy for mounting tissues of mosquito midgut that improved cell viability and techniques to administer SiR-actin with other suitable dye combinations to label the cells in the midgut of the mosquito during feeding. These techniques allowed for a full in vivo live-tissue imaging of parasitic invasion of the ookinete midgut for several hours.
Summary and Future Investigation
Eradicating malaria has required a multilayered approach by employing insecticides, antimalarial drugs, and insecticide-treated mosquito nets to reduce incidence. In addition, the first malaria vaccine RTS,S/AS01 is now available, but the data from clinical trials in children resulted in approximately 40% vaccine efficacy against malaria after four doses(15). These combined measures are estimated to have saved 7.6 million lives over the past two decades, but the recent WHO malaria report indicates a reduction in the number of cases may be stalling(2). Parasite resistance to antimalarial drugs and mosquito resistance to insecticides are two factors out of many that are contributing to the slower gains in controlling malaria.
In summary, these studies highlight how SiR-tubulin and SiR-actin in combination with new and established tools have aided researchers in better understanding cellular processes of the Plasmodium life cycle. With the advent of new laboratory techniques and probes, further biochemical and cellular studies of malaria will enhance our understanding of malaria.
Spirochrome Products
SiR-Actin Kit (Cat. # CY-SC001)
SiR-Tubulin Kit (Cat. # CY-SC002)
Cytoskeleton Kit (Includes SiR-Actin, SiR-Tubulin, and Verapamil) (Cat. # CY-SC006)
SiR-Lysosome Kit (Cat. # CY-SC012)
SiR700-Actin Kit (Cat. # CY-SC013)
SiR700-Tubulin Kit (Cat. # CY-SC014)
SiR700-DNA Kit (Cat. # CY-SC015)
SiR700-Lysosome Kit (Cat. # CY-SC016)
Flipper-TR Kit for fluorescence cell membrane microscopy (Cat # CY-SC020)
SPY555-Actin (Cat. # CY-SC202)
SPY555-Tubulin (Cat. # CY-SC203)