| Author | Title | Journal | Year | Article Link |
|---|
| Zhang, Jiahui et al. | Phillygenin prevents osteoclast differentiation and bone loss by targeting RhoA | Phytotherapy Research | 2024 | ISSN 1099--1573 |
| Bock, Fabian et al. | Rac1 promotes kidney collecting duct repair by mechanically coupling cell morphology to mitotic entry | Science advances | 2024 | ISSN 2375-2548 |
| Moztarzadeh, Sina et al. | Cortactin is in a complex with VE-cadherin and is required for endothelial adherens junction stability through Rap1/Rac1 activation | Scientific Reports 2024 14:1 | 2024 | ISSN 2045--2322 |
| González, Yasmilde Rodríguez et al. | PFTK1 kinase regulates axogenesis during development via RhoA activation | BMC Biology | 2023 | ISSN 1741-7007 |
| Ye, Qian et al. | Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice | Nature Communications 2023 14:1 | 2023 | ISSN 2041--1723 |
| Werder, Rhiannon B. et al. | The COPD GWAS gene ADGRG6 instructs function and injury response in human iPSC-derived type II alveolar epithelial cells | American journal of human genetics | 2023 | ISSN 1537--6605 |
| Toffali, Lara et al. | An isoform of the giant protein titin is a master regulator of human T lymphocyte trafficking | Cell reports | 2023 | ISSN 2211--1247 |
| Bartos, Katalin et al. | Renal FGF23 signaling depends on redox protein Memo1 and promotes orthovanadate-sensitive protein phosphotyrosyl phosphatase activity | Journal of Cell Communication and Signaling | 2023 | ISSN 1873-961X |
| Escuin, Sarah et al. | Dual mechanism underlying failure of neural tube closure in the Zic2 mutant mouse | DMM Disease Models and Mechanisms | 2023 | ISSN 1754-8411 |
| Dang, Iren et al. | Key role for Rac in the early transcriptional response to extracellular matrix stiffness and stiffness-dependent repression of ATF3 | Journal of Cell Science | 2023 | ISSN 1477-9137 |
| Martínez-Rendón, Jacqueline et al. | Ouabain Induces Transcript Changes and Activation of RhoA/ROCK Signaling in Cultured Epithelial Cells (MDCK) | Current Issues in Molecular Biology | 2023 | ISSN 1467-3045 |
| Rogg, Manuel et al. | A YAP/TAZ–ARHGAP29–RhoA Signaling Axis Regulates Podocyte Protrusions and Integrin Adhesions | Cells | 2023 | ISSN 2073-4409 |
| Choi, Hyehun et al. | LRRC8A anion channels modulate vascular reactivity via association with Myosin Phosphatase Rho Interacting Protein (MPRIP) | FASEB journal : official publication of the Federation of American Societies for Experimental Biology | 2023 | ISSN 1530-6860 |
| Lopes-Rodrigues, Vanessa et al. | AraC interacts with p75NTR transmembrane domain to induce cell death of mature neurons | Cell Death & Disease 2023 14:7 | 2023 | ISSN 2041--4889 |
| Zhai, Ruoyang et al. | Effects of sevoflurane on lung epithelial permeability in experimental models of acute respiratory distress syndrome | Journal of Translational Medicine | 2023 | ISSN 1479-5876 |
| Crespo, Grace Velez et al. | The Rac inhibitor HV-107 as a potential therapeutic for metastatic breast cancer | Molecular Medicine | 2023 | ISSN 1528-3658 |
| Morishita, Jun et al. | Identification of a small RhoA GTPase inhibitor effective in fission yeast and human cells | Open Biology | 2023 | ISSN 2046-2441 |
| Anastasaki, Corina et al. | Generation of human induced pluripotent stem cell-derived cerebral organoids for cellular and molecular characterization | STAR Protocols | 2022 | ISSN 2666--1667 |
| Zanin, Juan P. et al. | p75NTR prevents the onset of cerebellar granule cell migration via RhoA activation | eLife | 2022 | ISSN 2050-084X |
| Sánchez-de la Torre, Aníbal et al. | Cannabinoid CB1 receptor gene inactivation in oligodendrocyte precursors disrupts oligodendrogenesis and myelination in mice | Cell Death & Disease 2022 13:7 | 2022 | ISSN 2041--4889 |
| Weder, Bruce et al. | New Therapeutic Approach for Intestinal Fibrosis Through Inhibition of pH-Sensing Receptor GPR4 | Inflammatory Bowel Diseases | 2022 | ISSN 1078--0998 |
| Yadav, Vikas et al. | Increased MARCKS Activity in BRAF Inhibitor-Resistant Melanoma Cells Is Essential for Their Enhanced Metastatic Behavior Independent of Elevated WNT5A and IL-6 Signaling | Cancers | 2022 | ISSN 2072-6694 |
| Kumar, Akhilesh et al. | Essential role of Rnd1 in innate immunity during viral and bacterial infections | Cell Death & Disease 2022 13:6 | 2022 | ISSN 2041--4889 |
| Wang, Kankai et al. | PTBP1 knockdown promotes neural differentiation of glioblastoma cells through UNC5B receptor | Theranostics | 2022 | ISSN 1838-7640 |
| de Vallière, Cheryl et al. | pH-Sensing G Protein-Coupled Receptor OGR1 (GPR68) Expression and Activation Increases in Intestinal Inflammation and Fibrosis | International Journal of Molecular Sciences | 2022 | ISSN 1422-0067 |
| Meng, Zhipeng et al. | The Hippo pathway mediates Semaphorin signaling | Science Advances | 2022 | ISSN 2375-2548 |
| Darp, Revati et al. | Oncogenic BRAF induces whole-genome doubling through suppression of cytokinesis | Nature Communications 2022 13:1 | 2022 | ISSN 2041--1723 |
| Vadakumchery, Anila et al. | The Small GTPase RHOA Links SLP65 Activation to PTEN Function in Pre B Cells and Is Essential for the Generation and Survival of Normal and Malignant B Cells | Frontiers in Immunology | 2022 | ISSN 1664-3224 |
| Hauke, Michael et al. | Active RhoA Exerts an Inhibitory Effect on the Homeostasis and Angiogenic Capacity of Human Endothelial Cells | Journal of the American Heart Association | 2022 | ISSN 2047-9980 |
| Xue, Tan et al. | Effects of Aster B-mediated intracellular accumulation of cholesterol on inflammatory process and myocardial cells in acute myocardial infarction | Hellenic Journal of Cardiology | 2022 | |
| Francis, Caitlin R. et al. | Rab35 governs apicobasal polarity through regulation of actin dynamics during sprouting angiogenesis | Nature Communications 2022 13:1 | 2022 | ISSN 2041--1723 |
| Kumar, Akhilesh et al. | Essential role of Rnd1 in innate immunity during viral and bacterial infections | Cell Death & Disease | 2022 | Article Link |
| Ma, Yuanyuan et al. | Ror2-mediated non-canonical Wnt signaling regulates Cdc42 and cell proliferation during tooth root development | Development (Cambridge) | 2021 | ISSN 1477-9129 |
| Jozic, Ivan et al. | Glucocorticoid-mediated induction of caveolin-1 disrupts cytoskeletal organization, inhibits cell migration and re-epithelialization of non-healing wounds | Communications Biology | 2021 | ISSN 2399-3642 |
| Gurusamy, Malarvizhi et al. | G-protein-coupled receptor P2Y10 facilitates chemokine-induced CD4 T cell migration through autocrine/paracrine mediators | Nature Communications | 2021 | ISSN 2041-1723 |
| Zhou, Qun et al. | Inflammatory Immune Cytokine TNF-α Modulates Ezrin Protein Activation via FAK/RhoA Signaling Pathway in PMVECs Hyperpermeability | Frontiers in Pharmacology | 2021 | ISSN 1663-9812 |
| Porter, Lauren et al. | SUN1/2 Are Essential for RhoA/ROCK-Regulated Actomyosin Activity in Isolated Vascular Smooth Muscle Cells | Cells | 2020 | ISSN 2073-4409 |
| Krueger, Irena et al. | Reelin amplifies glycoprotein VI activation and alphaiib beta3 integrin outside-in signaling via PLC Gamma 2 and Rho GTPases | Arteriosclerosis, Thrombosis, and Vascular Biology | 2020 | ISSN 1524-4636 |
| Hasan, Wan Nuraini Wan et al. | Annatto-derived tocotrienol promotes mineralization of MC3T3-E1 cells by enhancing BMP-2 protein expression via inhibiting RhoA activation and HMG-CoA reductase gene expression | Drug Design, Development and Therapy | 2020 | ISSN 1177-8881 |
| Rong, Zhouyi et al. | Activation of FAK/Rac1/Cdc42-GTPase signaling ameliorates impaired microglial migration response to Aβ42 in triggering receptor expressed on myeloid cells 2 loss-of-function murine models | FASEB Journal | 2020 | ISSN 1530-6860 |
| Lachowski, Dariusz et al. | G Protein-Coupled Estrogen Receptor Regulates Actin Cytoskeleton Dynamics to Impair Cell Polarization | Frontiers in Cell and Developmental Biology | 2020 | ISSN 2296-634X |
| Salgado-Lucio, Monica L. et al. | FAK regulates actin polymerization during sperm capacitation via the ERK2/GEF-H1/RhoA signaling pathway | Journal of Cell Science | 2020 | ISSN 1477-9137 |
| Dias Gomes, Martim et al. | Polarity signaling ensures epidermal homeostasis by coupling cellular mechanics and genomic integrity | Nature Communications | 2019 | ISSN 2041-1723 |
| Majolée, Jisca et al. | CSN5 inhibition triggers inflammatory signaling and Rho/ROCK-dependent loss of endothelial integrity | Scientific Reports | 2019 | ISSN 2045-2322 |
| Santhana Kumar, Karthiga et al. | TGF-β Determines the Pro-migratory Potential of bFGF Signaling in Medulloblastoma | Cell Reports | 2018 | ISSN 2211-1247 |
| Li, Xu et al. | A positive feedback loop of profilin-1 and RhoA/ROCK1 promotes endothelial dysfunction and oxidative stress | Oxidative Medicine and Cellular Longevity | 2018 | ISSN 1942-0994 |
| Patra, Vijay Kumar et al. | The Skin Microbiome: Is It Affected by UV-induced Immune Suppression? | Frontiers in Microbiology | 2016 | ISSN 1664-302X |
| Lu, Wen Juan et al. | Senescence Mediated by p16INK4a Impedes Reprogramming of Human Corneal Endothelial Cells into Neural Crest Progenitors | Scientific Reports | 2016 | ISSN 2045-2322 |
| Yu, Yonghao et al. | Hydrogen-rich medium ameliorates lipopolysaccharide-induced barrier dysfunction via rhoa-mdia1 signaling in caco-2 cells | Shock | 2016 | ISSN 1540-0514 |
| López-Posadas, Rocío et al. | Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation | Journal of Clinical Investigation | 2016 | ISSN 1558-8238 |
| Rom, Slava et al. | The dual action of poly(ADP-ribose) polymerase -1 (PARP-1) inhibition in HIV-1 infection: HIV-1 ltr inhibition and diminution in Rho GTPase activity | Frontiers in Microbiology | 2015 | ISSN 1664-302X |
| Tang, Xiao et al. | HCLOCK Causes Rho-Kinase-Mediated Endothelial Dysfunction and NF-κ B-Mediated Inflammatory Responses | Oxidative Medicine and Cellular Longevity | 2015 | ISSN 1942-0994 |
| Manukyan, Arkadi et al. | A complex of p190RhoGAP-A and anillin modulates RhoA-GTP and the cytokinetic furrow in human cells | Journal of Cell Science | 2015 | ISSN 1477-9137 |
| Rom, Slava et al. | Poly(ADP-ribose) polymerase-1 inhibition in brain endothelium protects the blood-brain barrier under physiologic and neuroinflammatory conditions | Journal of Cerebral Blood Flow and Metabolism | 2015 | ISSN 1559-7016 |
| Herr, Michael J. et al. | Tetraspanin CD9 regulates cell contraction and actin arrangement via RhoA in human vascular smooth muscle cells | PLoS ONE | 2014 | ISSN 1932-6203 |
| Biechler V., Stefanie V. et al. | The impact of flow-induced forces on the morphogenesis of the outflow tract | Frontiers in Physiology | 2014 | ISSN 1664-042X |
| Mackay, Joanna L. et al. | Simultaneous and independent tuning of RhoA and Rac1 activity with orthogonally inducible promoters | Integrative Biology (United Kingdom) | 2014 | ISSN 1757-9708 |
| Chen, Xiaofei et al. | The TMEFF2 tumor suppressor modulates integrin expression, RhoA activation and migration of prostate cancer cells | Biochimica et Biophysica Acta - Molecular Cell Research | 2014 | ISSN 1879-2596 |
| Zhu, Ying Ting et al. | Knockdown of both p120 catenin and kaiso promotes expansion of human corneal endothelial monolayers via rhoa-rock-noncanonical BMP-NFκB pathway | Investigative Ophthalmology and Visual Science | 2014 | ISSN 1552-5783 |
| Papke, Christina L. et al. | Smooth muscle hyperplasia due to loss of smooth muscle α-actin is driven by activation of focal adhesion kinase, altered p53 localization and increased levels of platelet-derived growth factor receptor-β | Human Molecular Genetics | 2013 | ISSN 0964-6906 |
| Tan, Hong et al. | Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves | Developmental Biology | 2013 | ISSN 1095-564X |
| Kalia, Manjula et al. | Japanese Encephalitis Virus Infects Neuronal Cells through a Clathrin-Independent Endocytic Mechanism | Journal of Virology | 2013 | ISSN 0022--538X |
| Kanazawa, Yasushi et al. | The Rho-kinase inhibitor fasudil restores normal motor nerve conduction velocity in diabetic rats by assuring the proper localization of adhesion-related molecules in myelinating Schwann cells | Experimental neurology | 2013 | ISSN 1090--2430 |
| DiScipio, Richard G. et al. | Complement C3a signaling mediates production of angiogenic factors in mesenchymal stem cells | Journal of Biomedical Science and Engineering | 2013 | ISSN 1937--6871 |
| Chen, Guang et al. | Inhibition of chemokine (CXC motif) ligand 12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated cell migration by targeting mammalian target of rapamycin (mTOR) pathway in human gastric carcinoma cells (Journal of Biological Chemistry (2012) 2 | Journal of Biological Chemistry | 2012 | ISSN 0021-9258 |
| Greco, Carolina M. et al. | Chemotactic effect of prorenin on human aortic smooth muscle cells: a novel function of the (pro)renin receptor | Cardiovascular Research | 2012 | ISSN 0008--6363 |
| Dhaliwal, Anandika et al. | Cellular Cytoskeleton Dynamics Modulates Non-Viral Gene Delivery through RhoGTPases | | 2012 | PMID 22509380 |
| Ramsay, Alan G. et al. | Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenali******: Establishing a reversible immune evasion mechanism in human cancer | Blood | 2012 | ISSN 1528-0020 |
| Chen, Si Meng et al. | Inhibition of tumor cell growth, proliferation and migration by X-387, a novel active-site inhibitor of mTOR | Biochemical Pharmacology | 2012 | ISSN 0006-2952 |
| Howe, Grant A. et al. | RhoB controls endothelial cell morphogenesis in part via negative regulation of RhoA | Vascular Cell | 2012 | ISSN 2045--824X |
| Yang, Seungwon et al. | The RhoA-ROCK-PTEN pathway as a molecular switch for anchorage dependent cell behavior | Biomaterials | 2012 | ISSN 1878--5905 |
| Garrido‐Gómez, Tamara et al. | Annexin A2 is critical for embryo adhesiveness to the human endometrium by RhoA activation through F‐actin regulation | The FASEB Journal | 2012 | ISSN 0892--6638 |
| Zhou, Zhigang et al. | HSV-mediated gene transfer of C3 transferase inhibits Rho to promote axonal regeneration | Experimental Neurology | 2012 | ISSN 0014--4886 |
| McCoy, Kelly L. et al. | Protease-activated receptor 1 (PAR1) coupling to G(q/11) but not to G(i/o) or G(12/13) is mediated by discrete amino acids within the receptor second intracellular loop | Cellular signalling | 2012 | ISSN 1873--3913 |
| Ramseyer, Vanesa D. et al. | Tumor necrosis factor α decreases nitric oxide synthase type 3 expression primarily via Rho/Rho kinase in the thick ascending limb | Hypertension (Dallas, Tex. : 1979) | 2012 | ISSN 1524--4563 |
| Takefuji, Mikito et al. | G13-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure | Circulation | 2012 | ISSN 0009-7322 |
| Jin, Wanzhu et al. | Increased SRF transcriptional activity in human and mouse skeletal muscle is a signature of insulin resistance | Journal of Clinical Investigation | 2011 | ISSN 0021-9738 |
| Aguilar, Hector N. et al. | Phos-tag-based analysis of myosin regulatory light chain phosphorylation in human uterine myocytes | PLoS ONE | 2011 | ISSN 1932-6203 |
| Ganguly, Riya et al. | Adiponectin Increases LPL Activity via RhoA/ROCK-Mediated Actin Remodelling in Adult Rat Cardiomyocytes | Endocrinology | 2011 | ISSN 0013--7227 |
| Musso, Alessandra et al. | Relevance of the mevalonate biosynthetic pathway in the regulation of bone marrow mesenchymal stromal cell-mediated effects on T-cell proliferation and B-cell survival | Haematologica | 2011 | ISSN 1592--8721 |
| Lichtenstein, Mathieu P. et al. | Secretase-independent and RhoGTPase/PAK/ERK-dependent regulation of cytoskeleton dynamics in astrocytes by NSAIDs and derivatives | Journal of Alzheimer's disease : JAD | 2010 | ISSN 1875--8908 |
| Ridgway, Lon D. et al. | Modulation of GEF-H1 Induced Signaling by Heparanase in Brain Metastatic Melanoma Cells | Journal of cellular biochemistry | 2010 | ISSN 0730-2312 |
| Rapier, Rebecca et al. | The extracellular matrix microtopography drives critical changes in cellular motility and Rho A activity in colon cancer cells | Cancer Cell International | 2010 | ISSN 1475-2867 |
| Romero, Ana M. et al. | Chronic ethanol exposure alters the levels, assembly, and cellular organization of the actin cytoskeleton and microtubules in hippocampal neurons in primary culture | Toxicological Sciences | 2010 | ISSN 1096-6080 |
| Nini, Lylia et al. | Accurate and reproducible measurements of RhoA activation in small samples of primary cells | Analytical biochemistry | 2010 | ISSN 1096--0309 |
| Yang, Enyue et al. | Fluoride induces vascular contraction through activation of RhoA/Rho kinase pathway in isolated rat aortas | Environmental toxicology and pharmacology | 2010 | ISSN 1872--7077 |
| Hammar, Eva et al. | Role of the Rho-ROCK (Rho-Associated Kinase) Signaling Pathway in the Regulation of Pancreatic β-Cell Function | Endocrinology | 2009 | ISSN 0013--7227 |
| Chastre, Eric et al. | TRIP6, a novel molecular partner of the MAGI-1 scaffolding molecule, promotes invasiveness | The FASEB Journal | 2009 | ISSN 1530--6860 |
| Seifert, Jennifer L. et al. | Differential activation of Rac1 and RhoA in neuroblastoma cell fractions | Neuroscience letters | 2009 | ISSN 0304--3940 |
| Ramirez, Servio H. et al. | Activation of Peroxisome Proliferator-Activated Receptor γ (PPARγ) Suppresses Rho GTPases in Human Brain Microvascular Endothelial Cells and Inhibits Adhesion and Transendothelial Migration of HIV-1 Infected Monocytes | The Journal of Immunology | 2008 | ISSN 0022--1767 |
| Kinoshita, Nagatoki et al. | Apical Accumulation of Rho in the Neural Plate Is Important for Neural Plate Cell Shape Change and Neural Tube Formation | Molecular Biology of the Cell | 2008 | ISSN 1059-1524 |
| Mercer, Jason et al. | Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells | Science (New York, N.Y.) | 2008 | ISSN 1095--9203 |
| Sequeira, Linda et al. | Rho GTPases in PC-3 prostate cancer cell morphology, invasion and tumor cell diapedesis | Clinical & experimental metastasis | 2008 | ISSN 0262--0898 |
| Moore, Simon W. et al. | Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1 | Development | 2008 | ISSN 0950--1991 |
| Korobova, Farida et al. | Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells | Molecular biology of the cell | 2008 | ISSN 1939--4586 |
| Tanaka, Shigeru et al. | Neural expression of G protein-coupled receptors GPR3, GPR6, and GPR12 up-regulates cyclic AMP levels and promotes neurite outgrowth | The Journal of biological chemistry | 2007 | ISSN 0021--9258 |
| Schreibelt, Gerty et al. | Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling | FASEB journal : official publication of the Federation of American Societies for Experimental Biology | 2007 | ISSN 1530--6860 |
| Keely, Patricia J. et al. | Investigating integrin regulation and signaling events in three-dimensional systems | Methods in enzymology | 2007 | ISSN 0076--6879 |
| Rupp, Paul A. et al. | A role for RhoA in the two-phase migratory pattern of post-otic neural crest cells | Developmental Biology | 2007 | ISSN 0012-1606 |
| Bradley, William D. et al. | Integrin Signaling through Arg Activates p190RhoGAP by Promoting Its Binding to p120RasGAP and Recruitment to the Membrane | Molecular Biology of the Cell | 2006 | ISSN 1059-1524 |
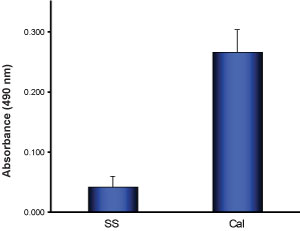
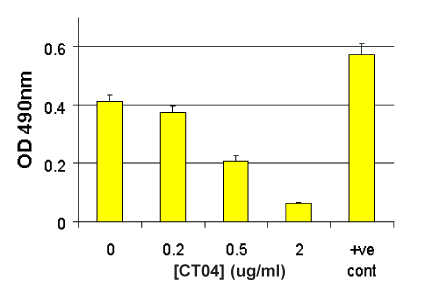
 G-LISA Activation Assay Technical Guide download here
G-LISA Activation Assay Technical Guide download here G-LISA Data Analysis (Absorbance) Excel Template download here.
G-LISA Data Analysis (Absorbance) Excel Template download here.