Tiam1 GEF Protein (DHPH Exchange Domain, aa1040-1406, MBP tag)
Product Uses
Tiam1 (T-cell lymphoma Invasion And Metastasis 1) is a guanine exchange factor with selectivity for Rac1, which mediates Rac1 activation under a variety of conditions and has been associated with multiple diseases (reviewed in 1, 2).
- Study inhibitors of Tiam1 GTP/GDP exchange activity
- Identification of Tiam1 DHPH domain binding proteins
- Study of Tiam1 GEF activity with different GTPases
Material
The DH/PH domain of human Tiam1 protein has been produced in a bacterial expression system. It contains an MBP fusion at its amino terminus. The accession number is NM_003253.2. The molecular weight of MBP-Tiam1 is approximately 86 kDa. The Tiam1 protein is supplied as a white lyophilized powder. Protein purity is determined by scanning densitometry of Coomassie Blue stained protein on a 4-20% polyacrylamide gradient gel. MBP-Tiam1 was determined to be 90% pure. (see Figure 1).
Figure 1. CS-GE04 SDS-PAGE Analysis
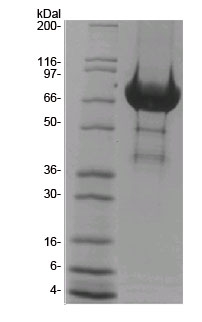
Legend: A 20 µg sample of recombinant Tiam1 (molecular weight approx. 86 kDa) was separated by electrophoresis in a 4-20% SDS-PAGE system and stained with Coomassie Blue. Protein quantitation was determined using the Precision Red Protein Assay Reagent (Cat. # ADV02). Mark12 molecular weight markers are from Life Technologies Inc.
Purity
Protein purity is determined by scanning densitometry of Coomassie blue stained protein on a 4-20% polyacrylamide gradient gel. The Tiam1 DHPH protein was determined to be >90% pure. (see Figure 1).
Storage
Before reconstitution, briefly centrifuge to collect the product at the bottom of the tube. The protein should be reconstituted to 5 mg/ml with ice cold nanopure water (20 µl water per 100 µg protein). When reconstituted, the protein will be in the following buffer: 20 mM Tris pH 7.5, 50 mM NaCl, 1 mM MgCl2, 5% (v/v) sucrose and 1% (v/v) dextran. In order to maintain high biological activity of the protein it is strongly recommended that the protein solution be supplemented with DTT to 1 mM final concentration, aliquoted into "experiment sized" amounts, snap frozen in liquid nitrogen and stored at -70°C. The protein is stable for six months if stored at -70°C. The protein must not be exposed to repeated freeze-thaw cycles. The lyophilized protein is stable at 4°C desiccated (<10% humidity) for one year. Further Tiam1 dilutions should be made in 20 mM Tris pH 7.5, 50 mM NaCl, 1 mM MgCl2 (not supplied).
Biological Activity Assay
The biological activity of Tiam1 DHPH can be determined from its ability to catalyze nucleotide exchange on Rac1 using the nucleotide exchange assay of Bodipy-GDP for excess GDP or GTP. Rac1 protein is pre-loaded with Bodipy-FL-GDP by adding excess EDTA e.g. 0.7 mmol EDTA per mmol Mg2+ ions present in the reaction. This sub-stock solution is then used in a dissociation assay format which indicates competition for exchange site with unlabeled nucleotide. The reaction is monitored by fluorescence measurement at 485nm Ex / 535nm Em. Stringent quality control ensures that the exchange rate of Bodipy-GDP or mant-GDP is enhanced at least five fold in the presence of 0.8 µM Tiam1 DHPH.
Reagents
1. MBP-Tiam1 DH/PH protein (Cat. # CS-GE04)
2. Rac1 protein (Cat. # RC01, CS-RC02 or CS-RC03)
3. Exchange buffer 2 (20 mM Tris-HCl pH 7.5, 50 mM NaCl, 1 mM DTT, 2 mM EDTA, 100 µg/ml BSA, and 0.75 µM Bodipy-FL-GDP), note - make fresh.
4. 50 mM MgCl2 in 20 mM Tris-HCl pH 7.5, 50 mM NaCl.
5. 5 mM GTP in 20 mM Tris-HCl pH 7.5, 50 mM NaCl.
Equipment
1. Fluorescence spectrometer (λex=485nm, λem=525nm, both with<15nm bandwidth)
2. Corning 96-well half area plates (Cat # 3686) or other plate with low protein binding surface.
Method
1. Place Tiam1 vial on ice and dilute to 0.40 µg/µl (8 µM) with ice cold Exchange Buffer.
2. Dilute Rac protein to 1.25 µg/µl (50 µM) with ice cold Exchange Buffer..
3. Add the following components together in a fresh 15 ml Falcon tube and mix well by pipetting or gentle vortex:
Component µl per well
Exchange Buffer 75
50 µM Rac 5
8 µM Tiam1 10
Note: For a total mixture volume, multiply the volume of reagents per well by the number of wells in the experiment, plus add 20% volume for pipetting losses.
4. Incubate for 20 min at room temperature (RT).
5. Lock in the nucleotide by adding 10 µl (per well) of 50 mM MgCl2.
6. Set up the fluorimeter with Excitation wavelength at 485 nm +/-15 nm and emission wavelength at 525 nm +/- 15 nm at RT.
7. Aliquot the pre-loaded mixture to the assigned wells and place the plate in the fluorimeter.
8. After 5 cycles (150 seconds), place the program on Hold or Pause, and remove the plate.
9. Pipette 10 µl of a) 5 mM GTP solution, b) a small compound, c) a test protein, d) 4 mM EDTA (+ve exchange control) or e) Dilution Buffer (negative control) in respective wells and immediately pipette up and down twice and resume reading for 20 minutes.
10. Save the readings after the kinetic protocols are finished. The exchange rate can be calculated by reducing the data to max slope (using 12 pts) or Vmax with the software that accompanies the plate reader. The exchange curve can be achieved by export to Microsoft Excel.
Figure 2. Tiam1 DHPH GEF protein domain mediated Bodipy-FL-GDP dissociation from Rac1.
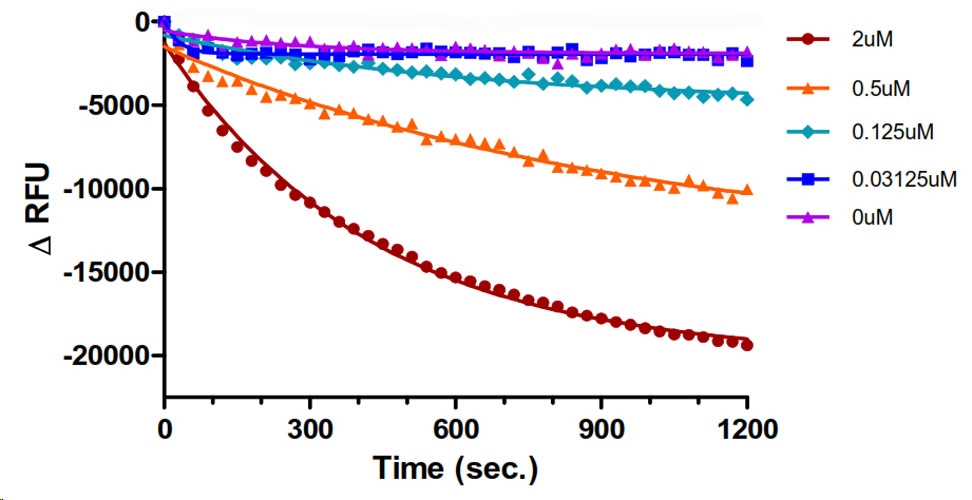
Legend: Rac1 protein (Cat. # RC01) (2.5 µM) was pre-loaded with Bodipy-FL-GDP using EDTA for exchange. The nucleotide was locked in place with excess Mg2+. Tiam1 at different concentrations, as shown, or Dilution Buffer (purple) was pipetted into wells of a black 384-well low volume plate. At time zero, 500 µM GTP was pipetted in to the wells and the reactions were monitored for 20 min by reading every 30 sec.
For product Datasheets and MSDSs please click on the PDF links below.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
Q1. What formats are available to study nucleotide exchange on small G-proteins?
A1. Several types of assay exist, these range from radio-labeled nucleotide filter based assays, to fluorescence enhancement using fluorescent nucleotide conjugates such as mant-GTP or -GDP, or Bodipy-FL-GTP or-GDP. These reporters enhance their fluorescence when binding to the small G-protein and hence the fluorescent signal is proportional to the amount of reporter-occupied nucleotide binding sites.
Q2. Which is best to measure nucleotide association or dissociation?
A2. Either can be used to indicate general nucleotide exchange, and the preference lies in whether you are looking for exchange for activation purposes, for example in the case of tumor physiology Ras GEFs are up-regulated which increases the GTP bound state of the small G-protein, thus in drug development efforts its important to know the rate of GDP dissociation and replacement with GTP, which is assumed to correlate with activation in cells.
Q3. What should be titrated to achieve a useful GTP exchange assay?
A3. The two factors that affect the signal to noise of a GEF assay are the proteins themselves, usually GEFs are tritrated from 50 nM to 5 µM, and small G-proteins from 0.5 to 5 µM.
If you have any questions concerning this product, please contact our Technical Service department at tservice@cytoskeleton.com
