Tubulin Tools
Since 1993 Cytoskeleton has provided purified tubulin proteins to the scientific community. We continually strive to provide the purest, most biological active and relevant tubulin proteins, kits and reagents for today's researchers.
The product range includes tubulins from fungal to human origin, fluorescent and biotinylated conjugants, and user friendly kits. The kits are designed to enhance the productivity ot the time you have available for research, usually saving months of development time. In addition, we provide tubulin antibodies which are not provided by generic antibody providers.
Learn More About Tubulin and Drug Development
Our most recent Tubulin writings can be found below. However Cytoskeleton has been writing about Tubulin for years, and that library of information can be found here
Motor Control: How Kinesins Drive Mitosis
Kinesins were first discovered in 1985 when it was observed that a new class of ATPases could cycle on and off microtubules and induce organelle movement along the axons of squid and vertebrate brain2,3. Since then, 45 kinesin proteins have been identified in humans, with the Kinesins superfamily (KIF) organized into 14 recognized kinesin families4. Most kinesins have a plus-end directed motility, but motors in the kinesin-14 subfamily have a C-terminal motor and are minus-end directed. The general architecture of kinesins consists of three domains: 1) a catalytic motor or head domain that is well conserved within each kinesin family that can hydrolyze ATP and binds microtubules, 2) an adjacent neck domain that is involved in coiled-coil interactions and can organize the motor into higher-order oligomers, 3) On the opposite end of the protein is the tail domain, which can be highly divergent within a kinesin family, and is sometimes used to interact with cargo proteins, DNA, or regulatory domains5–8. Below, we will look at recent findings on how kinesins affect the formation of the mitotic spindle and the organization of the chromosomes through the various stages of mitosis9.
The Structure Of Microtubules - A Brief History
The first images of tubulin within the cell began to be observed in the 1950s and 1960s via transmission electron microscopy(1). However, initial fixation methods at that time did not preserve the tubulin structure for it to be observed consistently. Initially, scientists thought these tubulin structures were canaliculi, endoplasmic reticulum, or filamentous elements(2,3). In 1963, after the development of using glutaraldehyde as a fixative, the consistency and resolution of tubulin improved, and the term “microtubules” (MTs) was introduced(4-6). Several years later, Gary Borisy and Ed Taylor at the University of Chicago used tritium-labeled colchicine as a small molecule probe to elucidate how colchicine inhibited mitosis. They discovered that a structural protein must be binding the labeled colchicine, as it was taken up by KB and Hela cells in tissue culture(7), sea urchin eggs(8), sea urchin sperm tails(9), and the brain(10). Soon after, Hideo Mohri gave the colchicine-binding protein the official name of tubulin(11).
Tubulin Tools In Action - Citation Spotlights
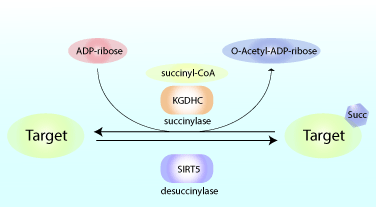
Altered Succinylation Of Mitochondrial Proteins, APP And Tau In Alzheimer’s Disease
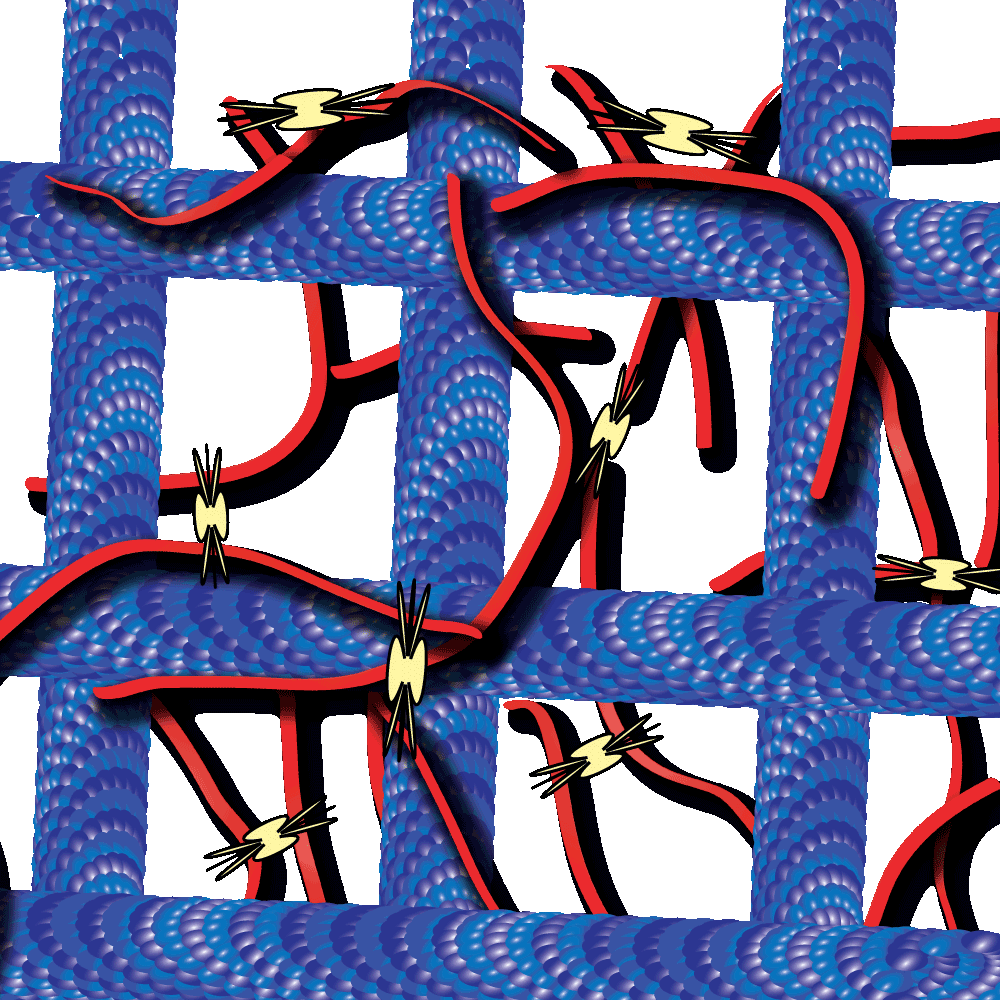
Myosin-driven actin-microtubule networks exhibit self-organized contractile dynamics
Reduced glucose metabolism in specific brain regions has been shown to correlate with clinical cognitive dysfunction, and preclinical research studies showed that reduced glucose metabolism can amplify learning and memory deficits. As a result, there is increasing interest in defining the precise metabolic pathways involved in the pathogenesis of neurological diseases like Alzheimer’s disease (AD). A recent study by Yang et al. showed that succinylation, a metabolism-associated post-translational protein modification, provides a potential link between abnormal metabolism and AD pathology. Proteomic analysis of brain tissue from AD and control patients showed interesting and defined changes in succinylation profiles in AD patients. Succinylation of multiple proteins in the mitochondria, an organelle rich in succinylated proteins, declined in patients with AD. Surprisingly, in AD patients, succinylation of a small number of cytosolic proteins increased, and two targets with the largest increases were amyloid precursor protein (APP) and microtubule-associated tau.
Cytoskeleton proteins, actin and tubulin, are two of the most well-characterized proteins and the networks they form have been actively studied over the past decade. Interestingly, these actin and tubulin networks interact extensively in cells, yet our understanding of how these networks affect the other’s dynamics is just beginning to come to light. Work by Dr. Ross and Dr. Robertson-Anderson has led the way towards unmasking this mystery. In their recent study these groups sought to determine how the interactions between actin and microtubule networks impact actomyosin activity. They utilized cutting-edge technologies like particle image velocimetry and dynamic differential microscopy to measure the network dynamics of actin and microtubules that are co-entangled in the presence of myosin II. Interestingly they observed that microtubules facilitated organized contraction of actomyosin networks that were otherwise disjointed and displayed disordered dynamics. Importantly, they also observed that these co-entangled networks can undergo ballistic contraction with indistinguishable characteristics.
Please visit our product pages for product specific citations
99% Pure Tubulin Protein - Tubulin Polymerization Assay - Pre-Formed Microtubules
Cytoskeleton's line of Tubulin Tools
Cytoskeleton Inc. was the first company to offer biologically active tubulin proteins, kits and reagents for the scientific community. Click through this brochure or the button below to see a sample of Cytoskeleton's Tubulin Tools
Have a technical question about our tools? Send us an email at tservice@cytoskeleton.com and our experts will anwser any question you may have!