ARNO (also known as cytohesin-2) is a guanine nucleotide exchange factor (GEF) that activates ADP-ribosylation factor (ARF) small GTPases, regulating membrane trafficking and actin cytoskeleton remodeling. It plays key roles in processes like cell migration, endocytosis, and signal transduction.
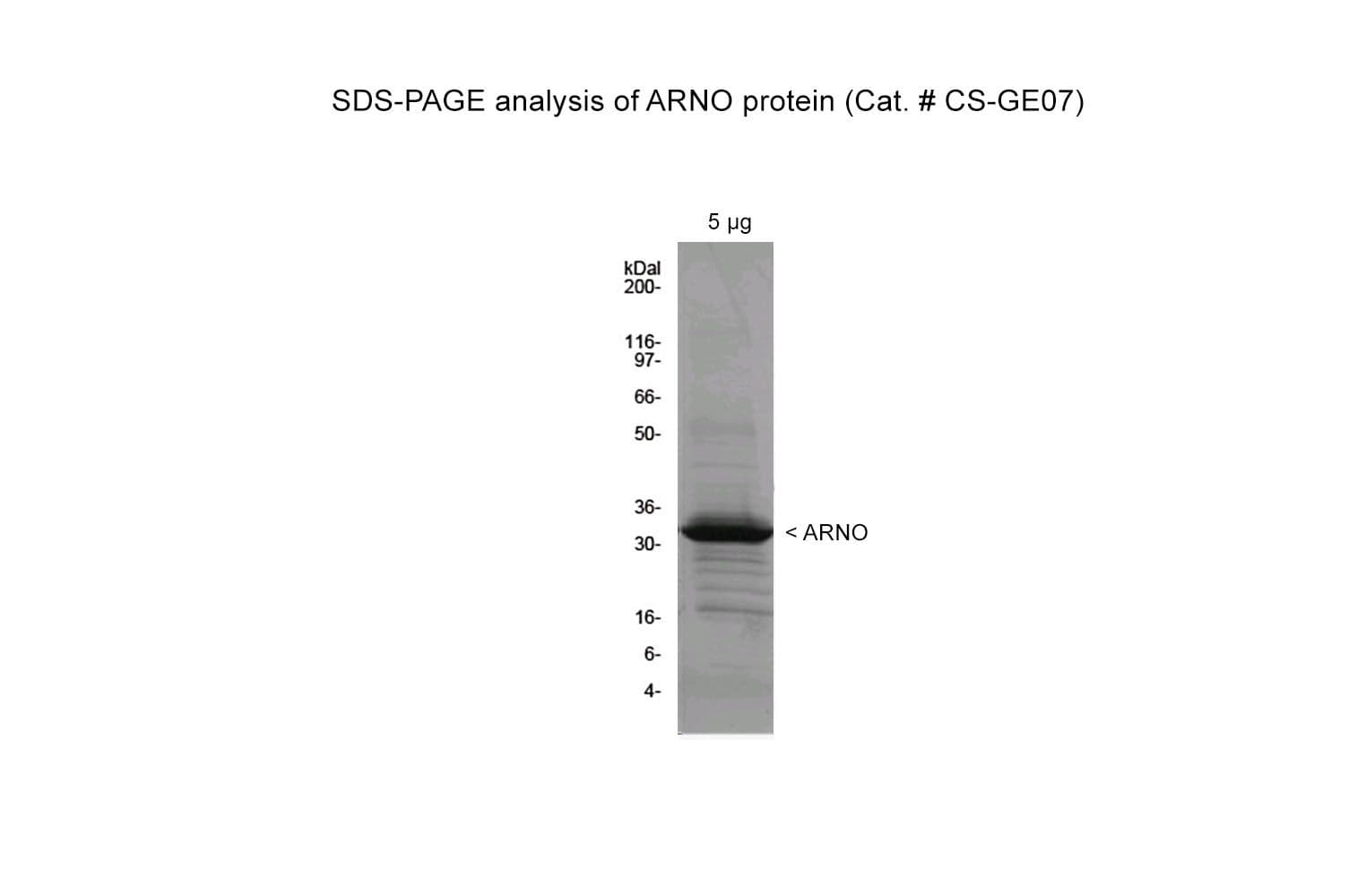
The Sec7 GEF domain (amino acids 31-267) of human ARNO protein has been produced in a bacterial expression system. It is also 6xHis tagged at its amino terminus for purification purposes. The fusion protein has a molecular weight of 33 kDa, and it is supplied as a lyophilized protein
Protein purity is assessed using scanning densitometry of Coomassie-stained SDS-PAGE gels. CS-GE07 is ≥90% pure
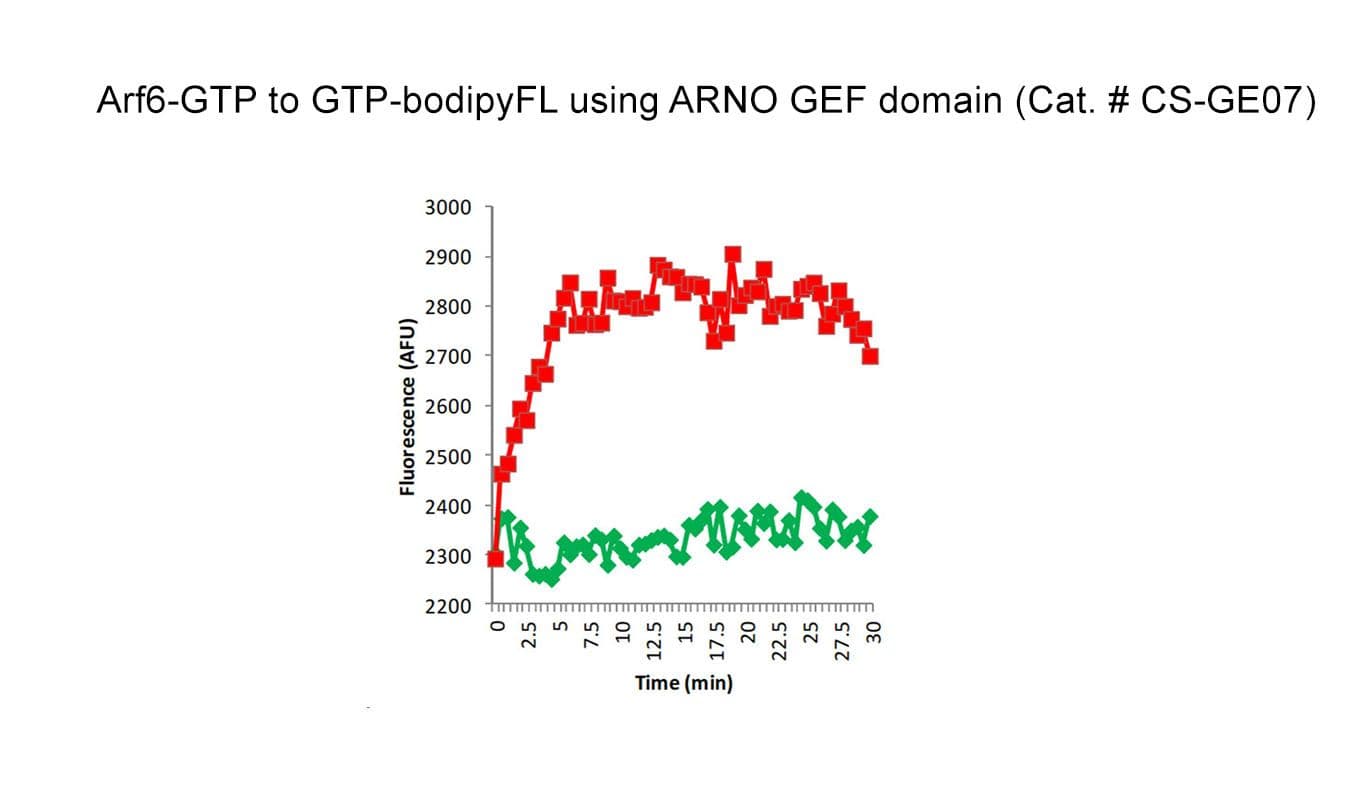
The biological activity of CS-GE07 is determined using an in vitro GEF assay. Under the experimental conditions (see datasheet), CS-GE07 increases Arf1 GTP exchange by ≥2 fold over intrinsic Arf1 exchange.
Cat. #CS-GE07