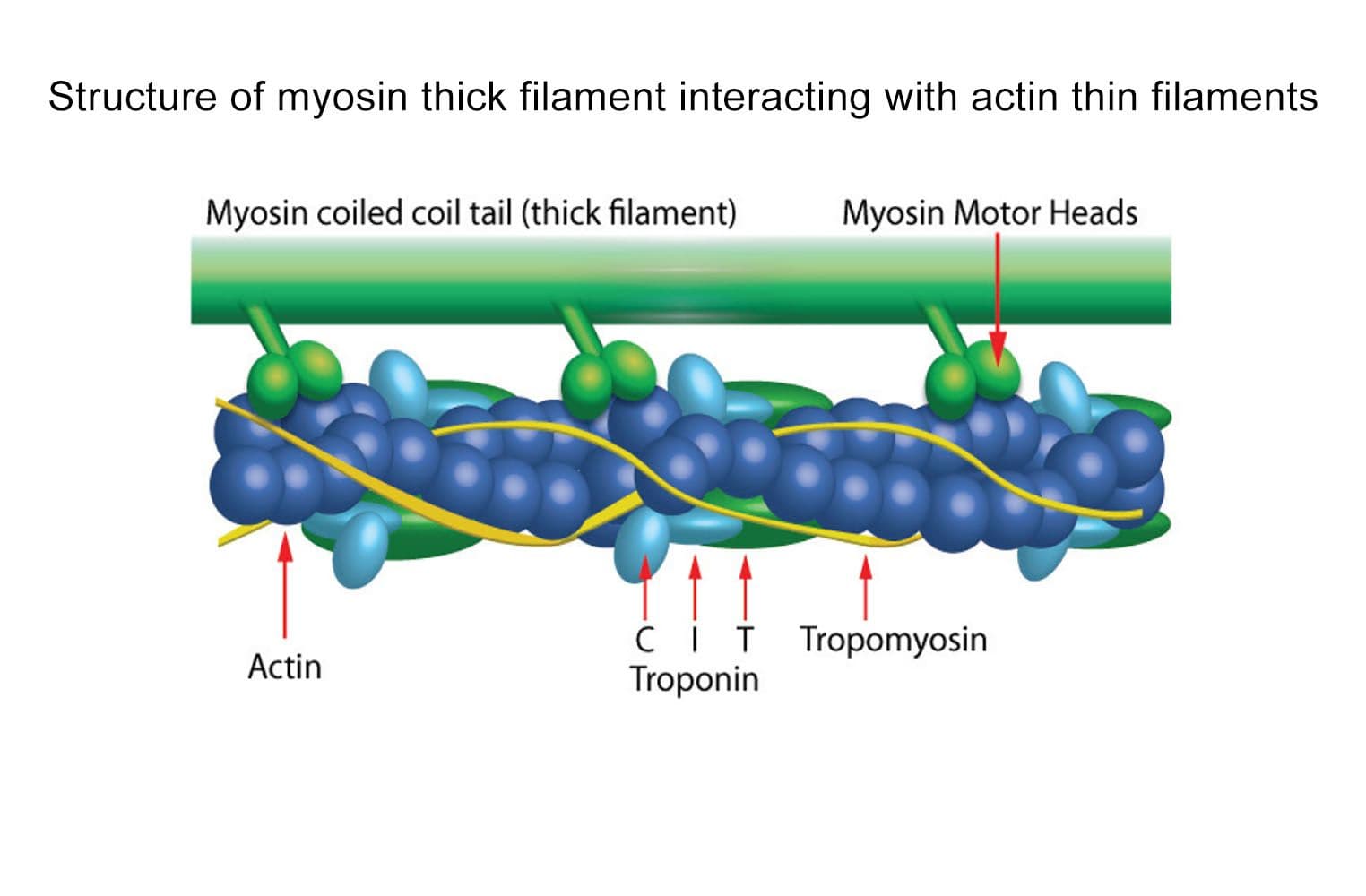
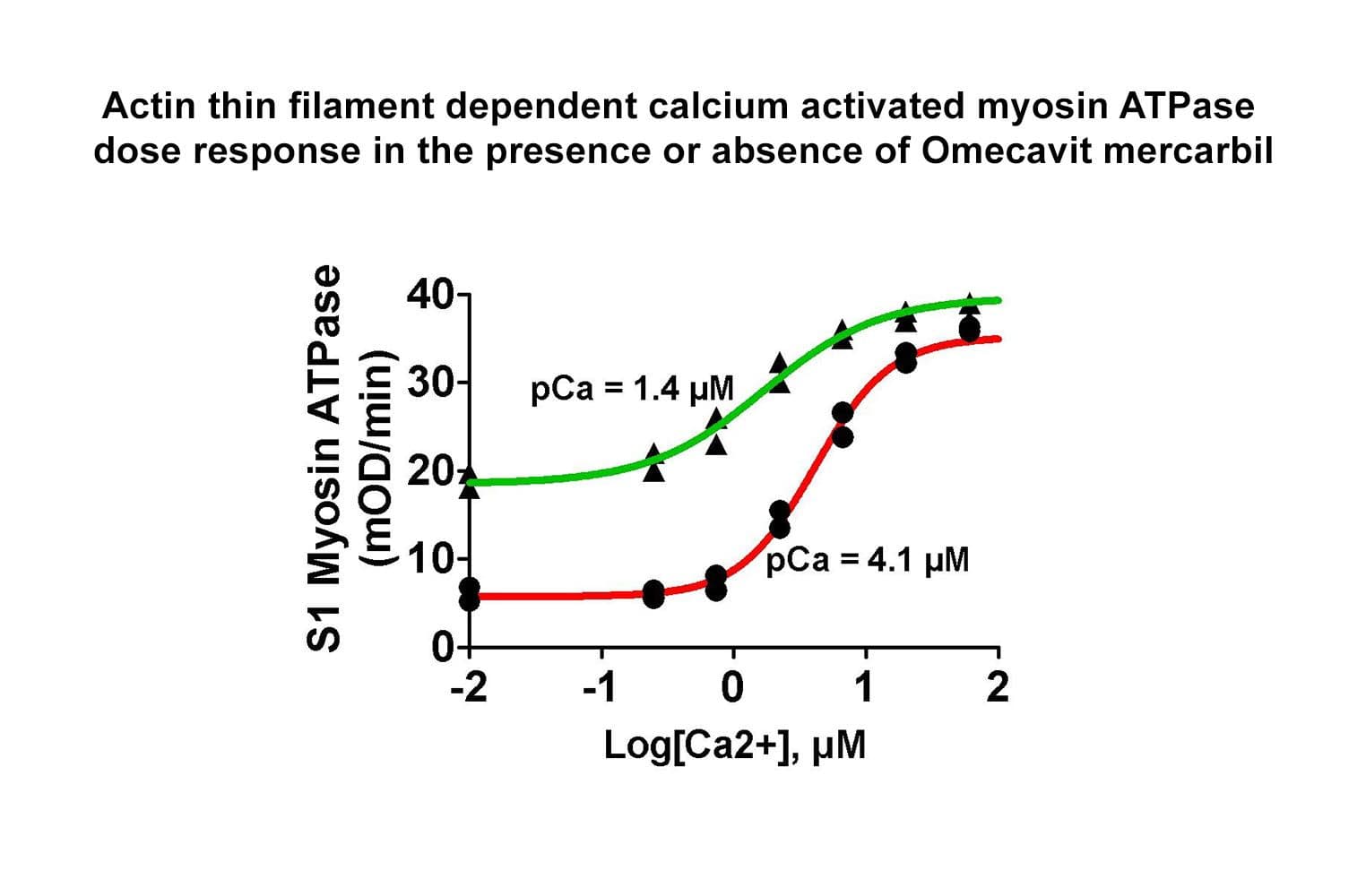

The biological activity of cardiac TF is determined from its ability to regulate myosin S1 ATPase activity. The TF re-creates coated filaments, which are analogous to the thin filaments of muscle fibers. Myosin S1 is added in sub-stoichiometric amounts, and the reaction is initiated with ATP and calcium. Stringent quality control ensures that in the absence of exogenous calcium, the TF complex completely inhibits myosin S1 ATPase. On addition of 20 M calcium, myosin S1 ATPase is restored. Calcium binds to Troponin C, which dissociates from F-actin, allowing myosin S1 to bind. A high-quality S1 myosin preparation is essential to obtain the best performance from the TF complex.
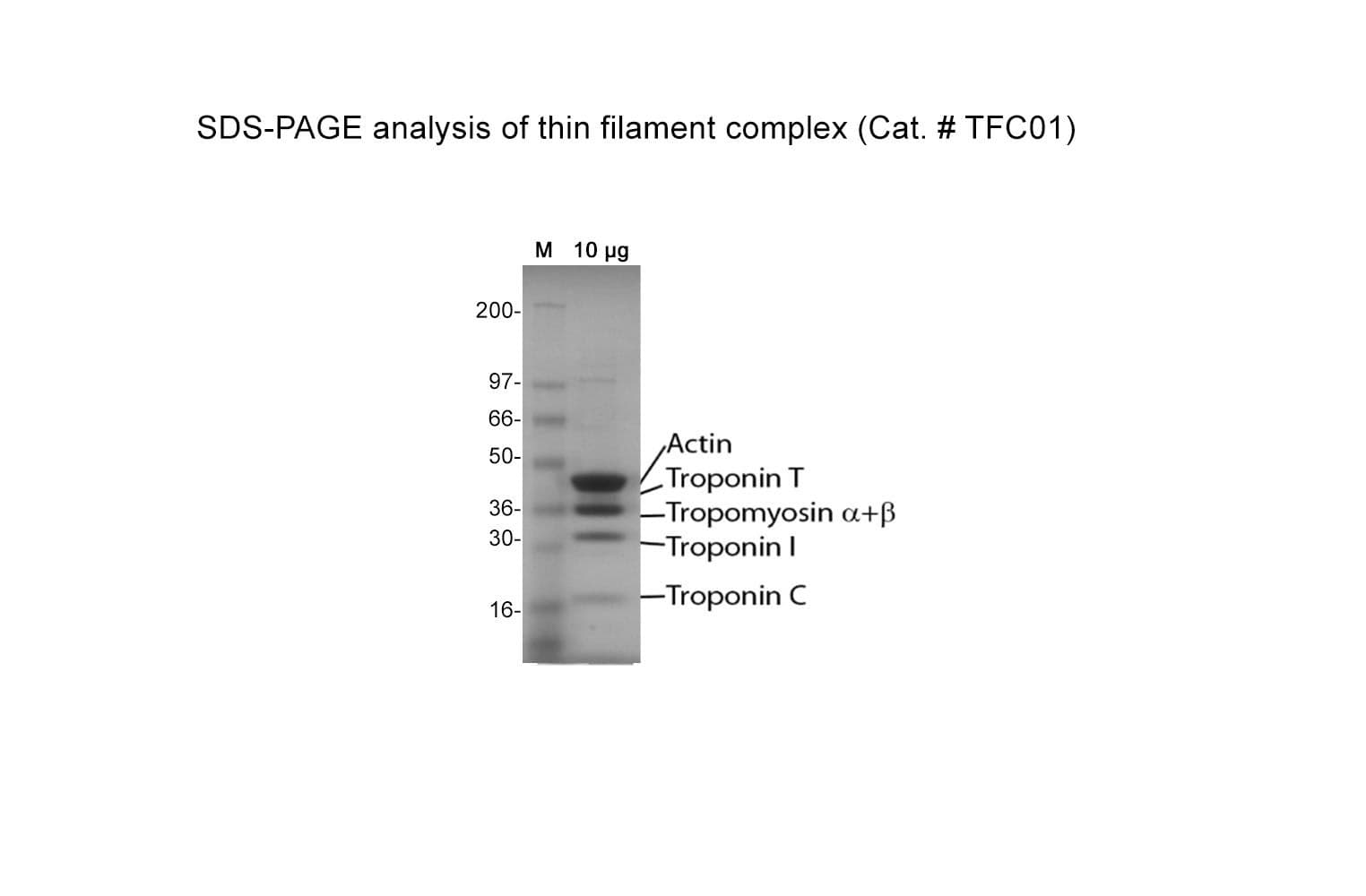
Protein purity is determined by scanning densitometry of Coomassie Blue-stained protein on a 4-20% gradient polyacrylamide gel. The purity of the cardiac TF complex is >95%.
The cardiac TF complex has been assembled from purified cardiac muscle F-actin Cat. # AD99 and Tropomyosin / Troponin protein Cat # TT05. The TF complex is composed of six proteins: Actin, Tropomyosin , Tropomyosin , Troponin C, Troponin I, and Troponin T, in a stoichiometric ratio of 7:1:1:1:1:1. The complex is supplied as a white lyophilized powder
Cat. #TFC01




© 2026 Cytoskeleton, Inc All Rights Reserved.