Affinity reagent for isolation of activated (GTP-bound) Rho protein in cell and tissue lysates. Requires 200 – 1000 µg lysate starting material per assay
The Rho-GTP binding domain (RBD) of the human Rhotekin protein has been overexpressed as a GST-tagged recombinant protein in a bacterial expression system and bound to colored glutathione Sepharose beads. The Rhotekin-RBD contains amino acids 7-89 of the Rhotekin protein and has an approximate molecular weight of 35 kDa. The protein is supplied as 2 mg of lyophilized bead-bound protein. The bead matrix is pink/purple for easy detection. One tube of RT02 is sufficient for approximately 30-40 assays.
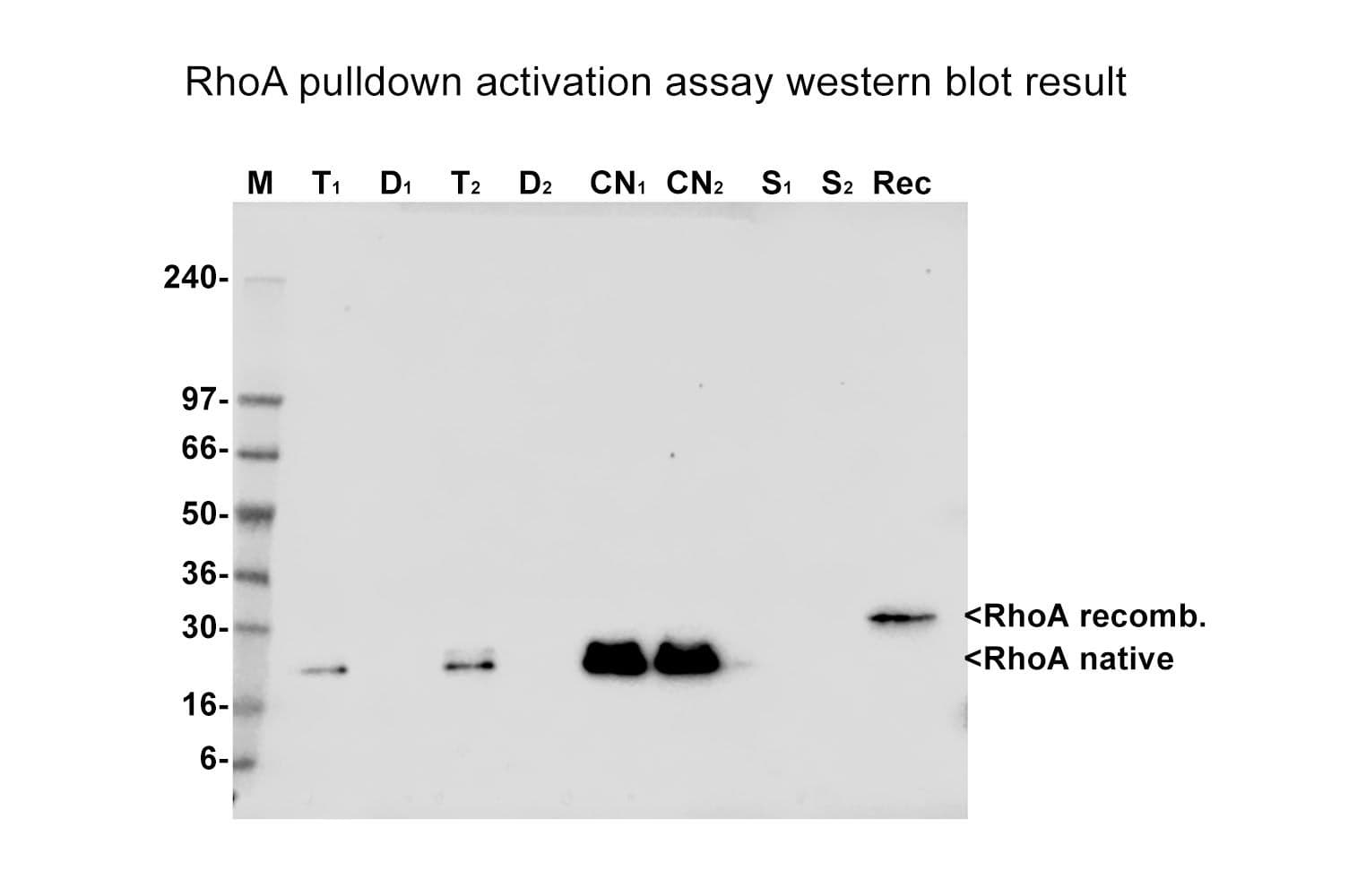
Rhotekin-RBD beads selectively bind the active (GTP-bound) forms of RhoA/B/C, showing minimal affinity for their inactive (GDP-bound) forms. A standard assay (20 µl beads, 300 µg lysate) demonstrates this selectivity by comparing GTPγS-loaded human platelet lysate to GDP-loaded lysate. After isolating activated RhoA/B/C, western blotting with a RhoA-specific antibody is performed. GTPγS, a non-hydrolysable GTP analog, locks Rho in its active conformation. Running GDP and GTPγS conditions in parallel validates both the specificity and performance of the beads. This assay results in a 3-5X enhancement of active RhoA signal in the GTP γS sample.
Cat. #RT02