+3
Loading...
Validated for:
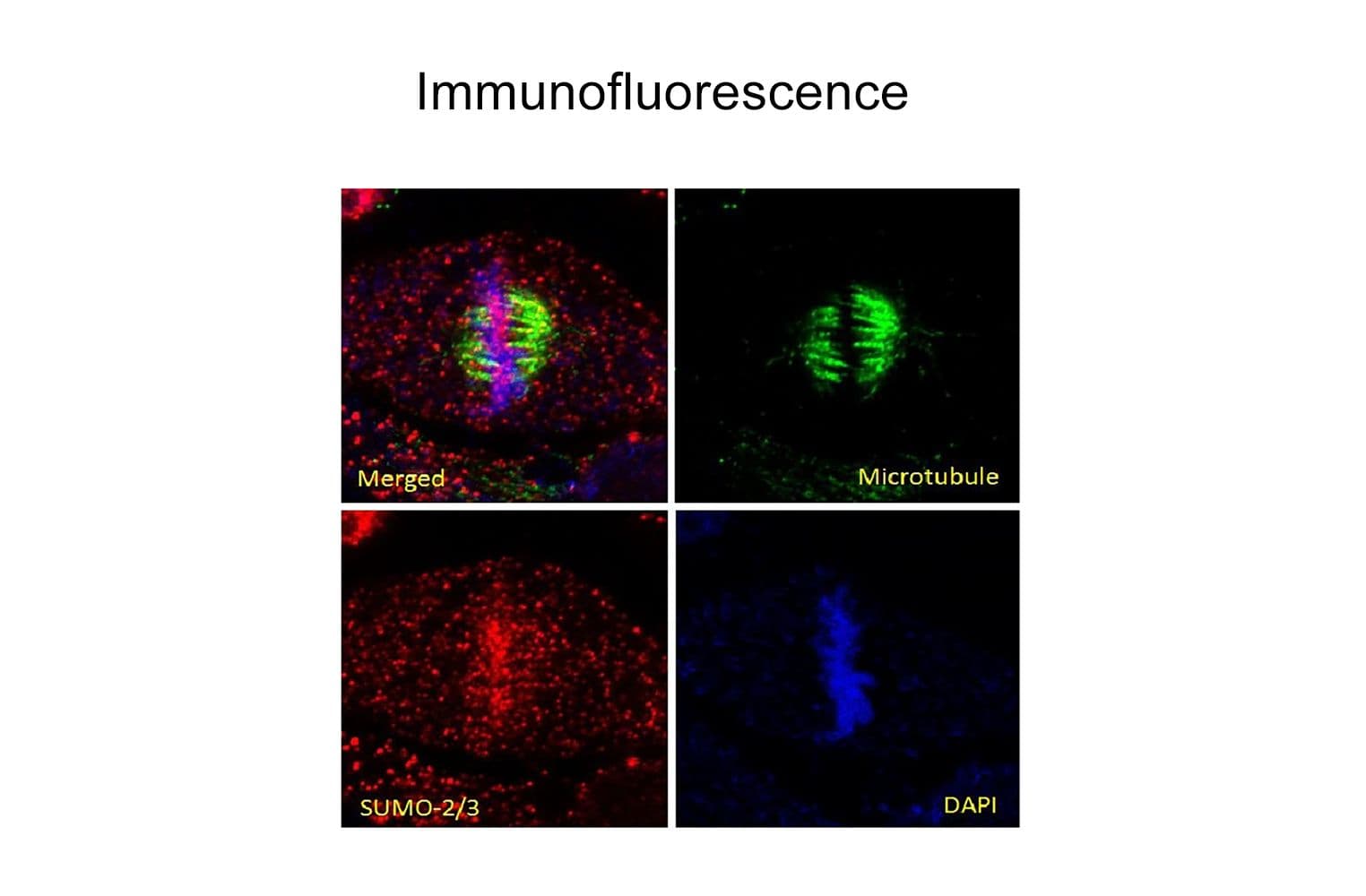
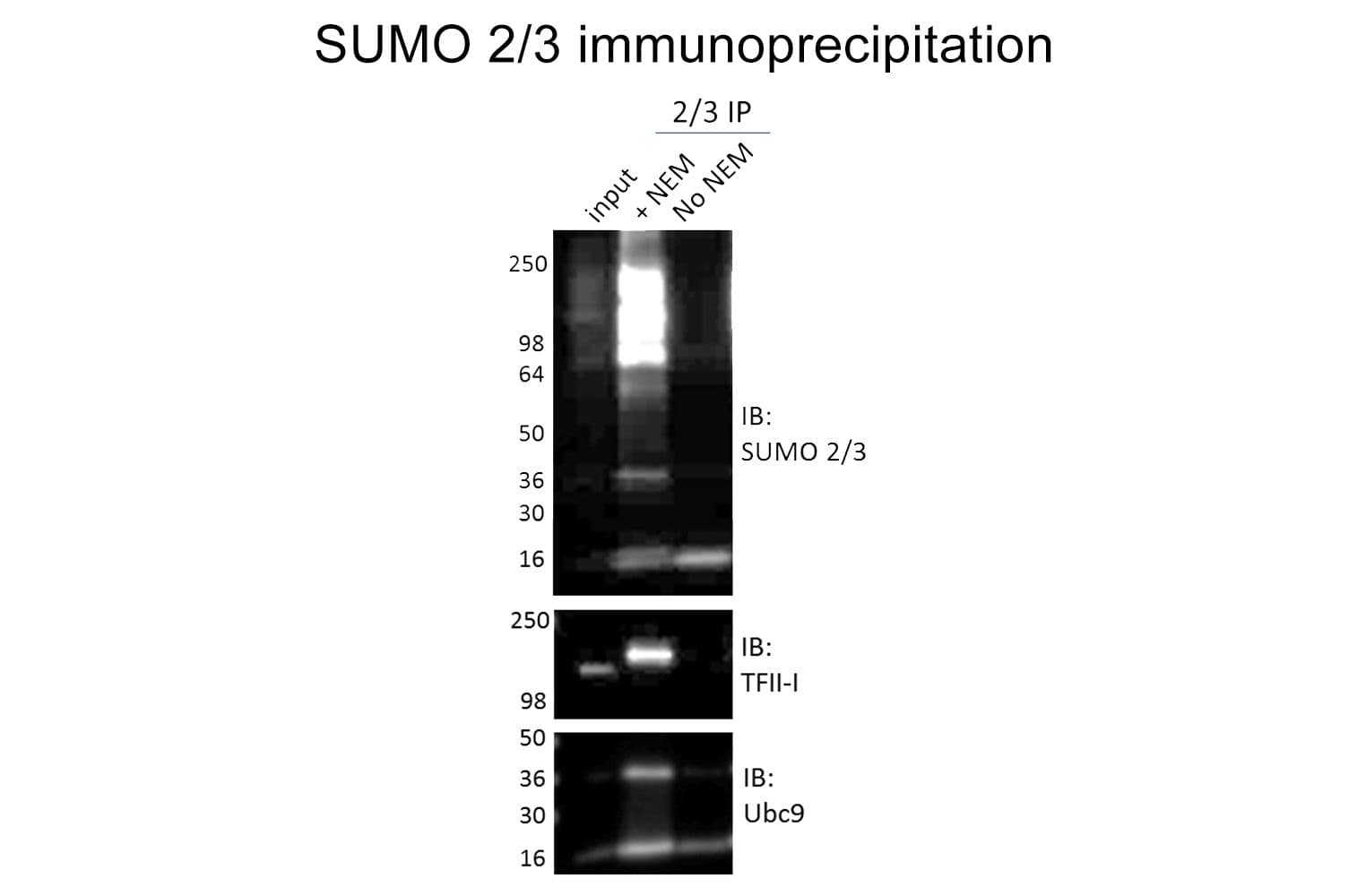
Anti-SUMO-2/3 antibody (ASM23) was raised against full-length recombinant SUMO-2 protein (Uniprot: P61956) combined with a proprietary mix of peptides that include CQIRFRFDGQPINE. Epitope mapping has identified that the antibody recognizes a sequence/structure within the peptide CQIRFRFDGQPINE. It detects a wide range of SUMO-2/3-targeted proteins. ASM23 is purified by Protein G affinity chromatography.
Host/isotype/clone:
Reactivity:
Amount of material:
Working concentrations:
Formulation
Sensitivity:
Specificity:
Cat. #ASM23