+3
Kit contents (sufficient for 100 x 1 ml lysates)
Equipment & materials required
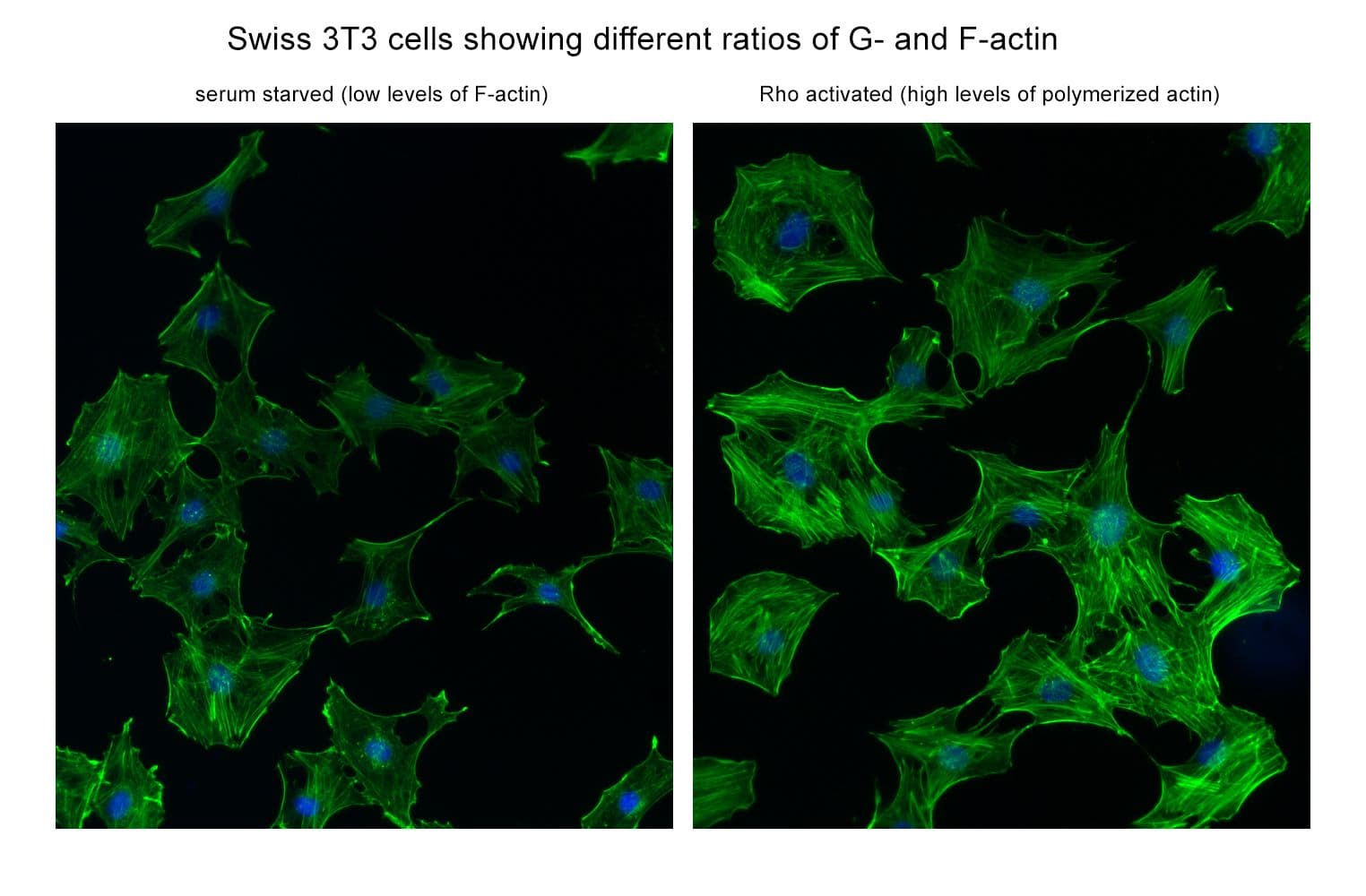
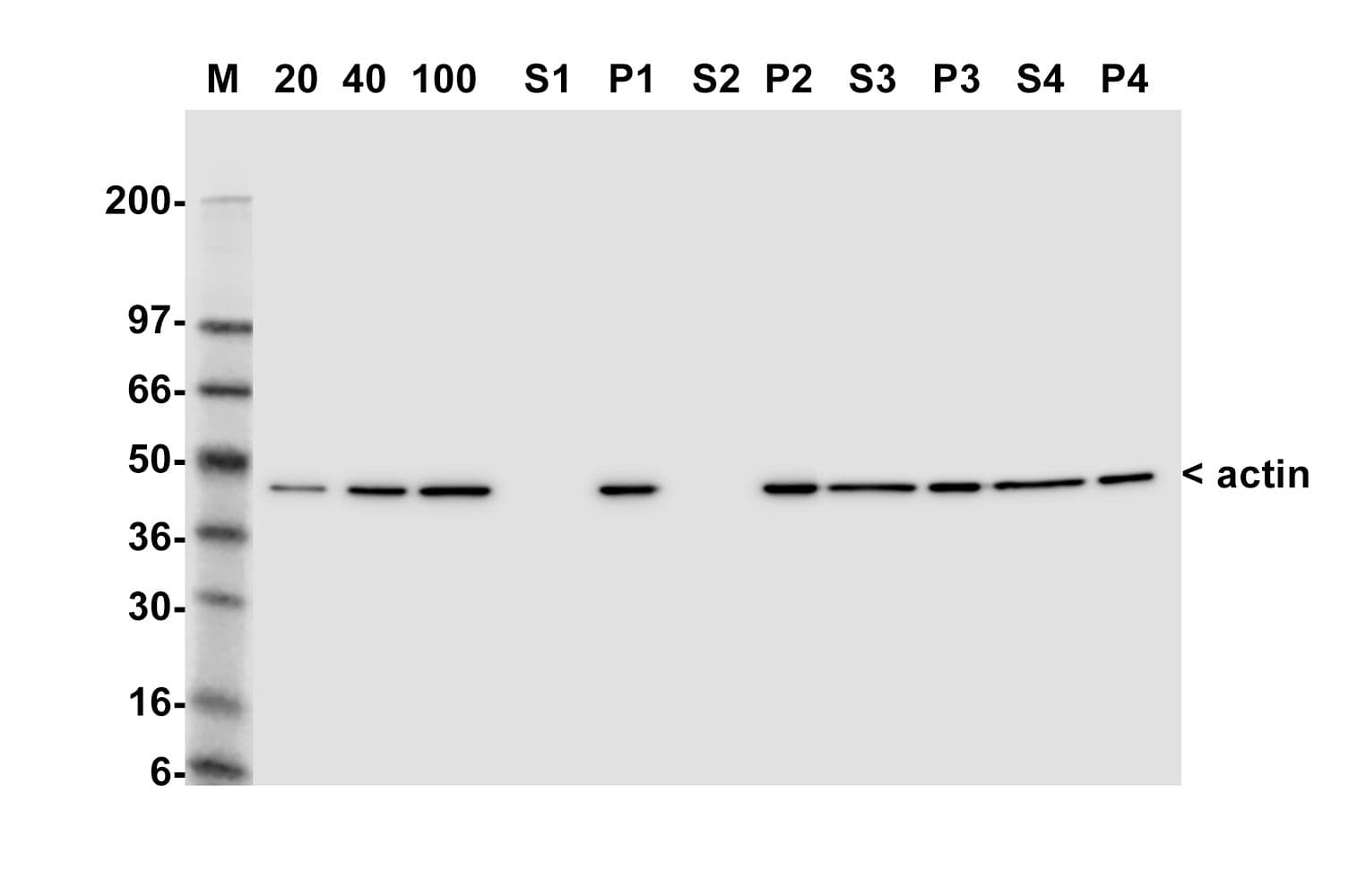
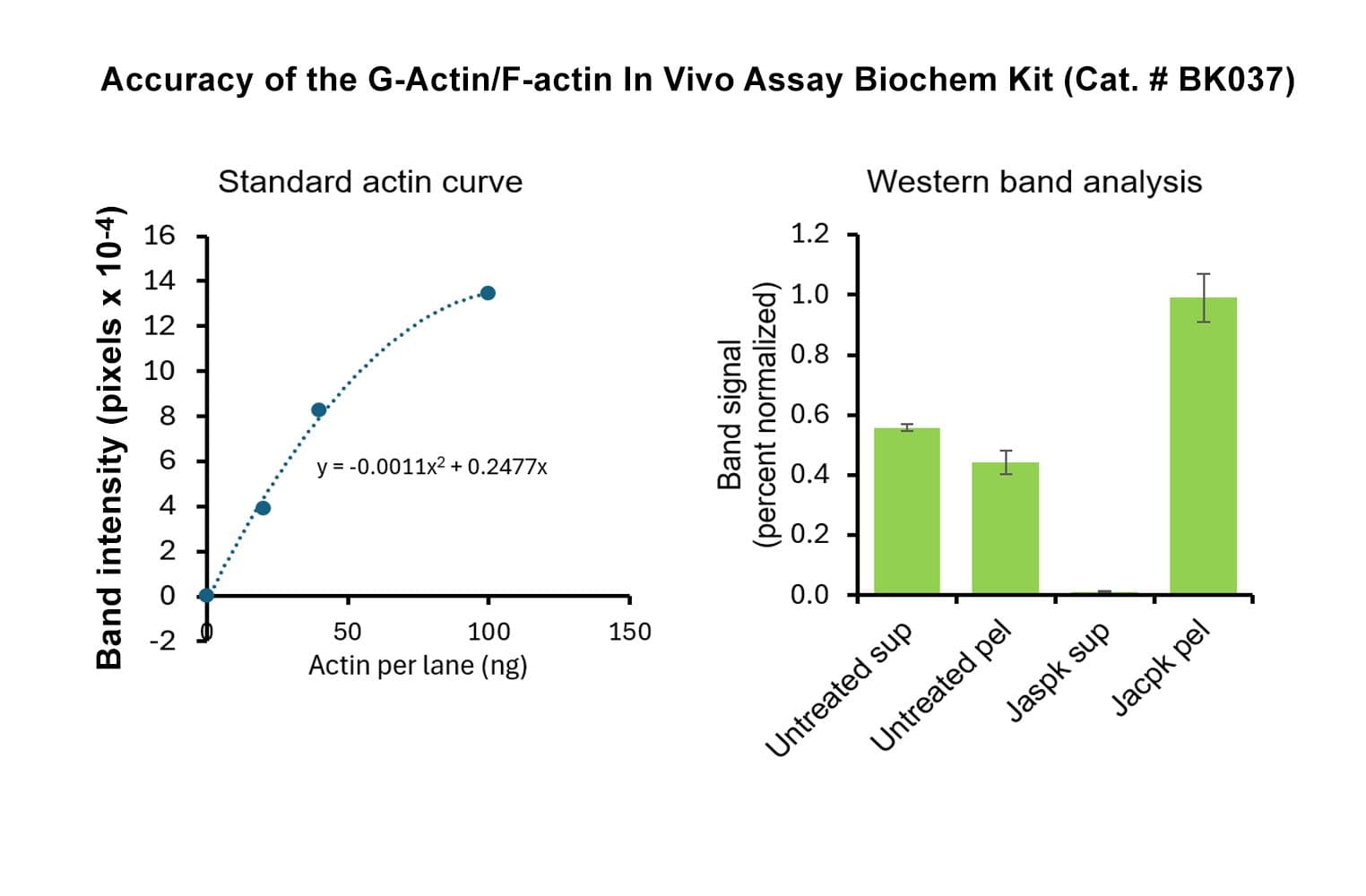
This kit enables in vivo measurement of the Filamentous: Globular (F: G) actin ratio in cells and tissues, providing insight into actin dynamics under set experimental conditions. Cells are lysed in a detergent-based buffer that preserves both G- and F-actin. The buffer solubilizes G-actin, while F-actin remains insoluble. Centrifugation separates the fractions, pelleting F-actin and leaving G-actin in the supernatant. Both fractions are analyzed by SDS-PAGE and quantified via Western blot.
Key characteristics
Cat. #BK037