+3
Kit contents (24 - 96 assays: strip-wells allow single or multiple assays per run)
Equipment & materials required
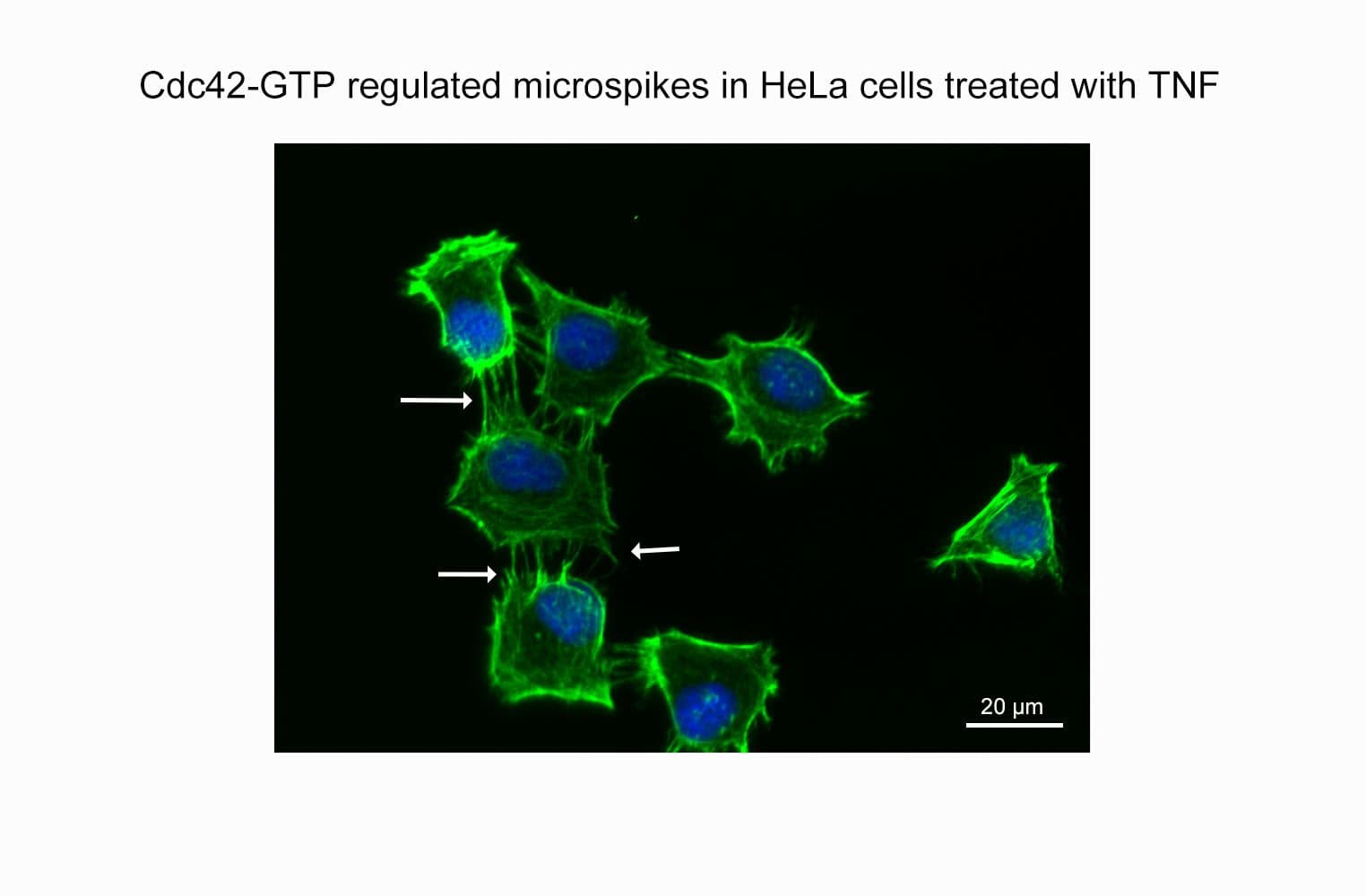
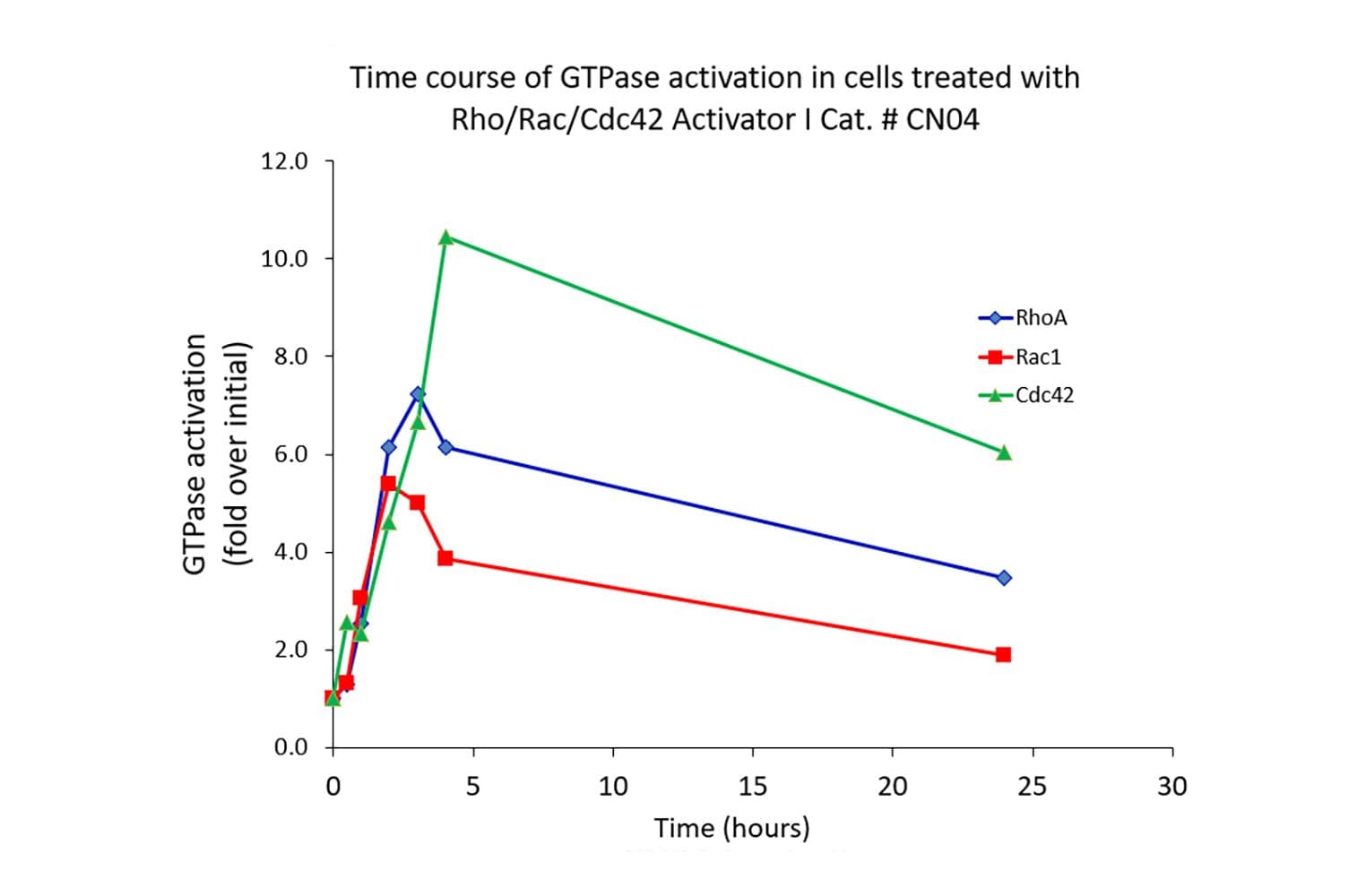
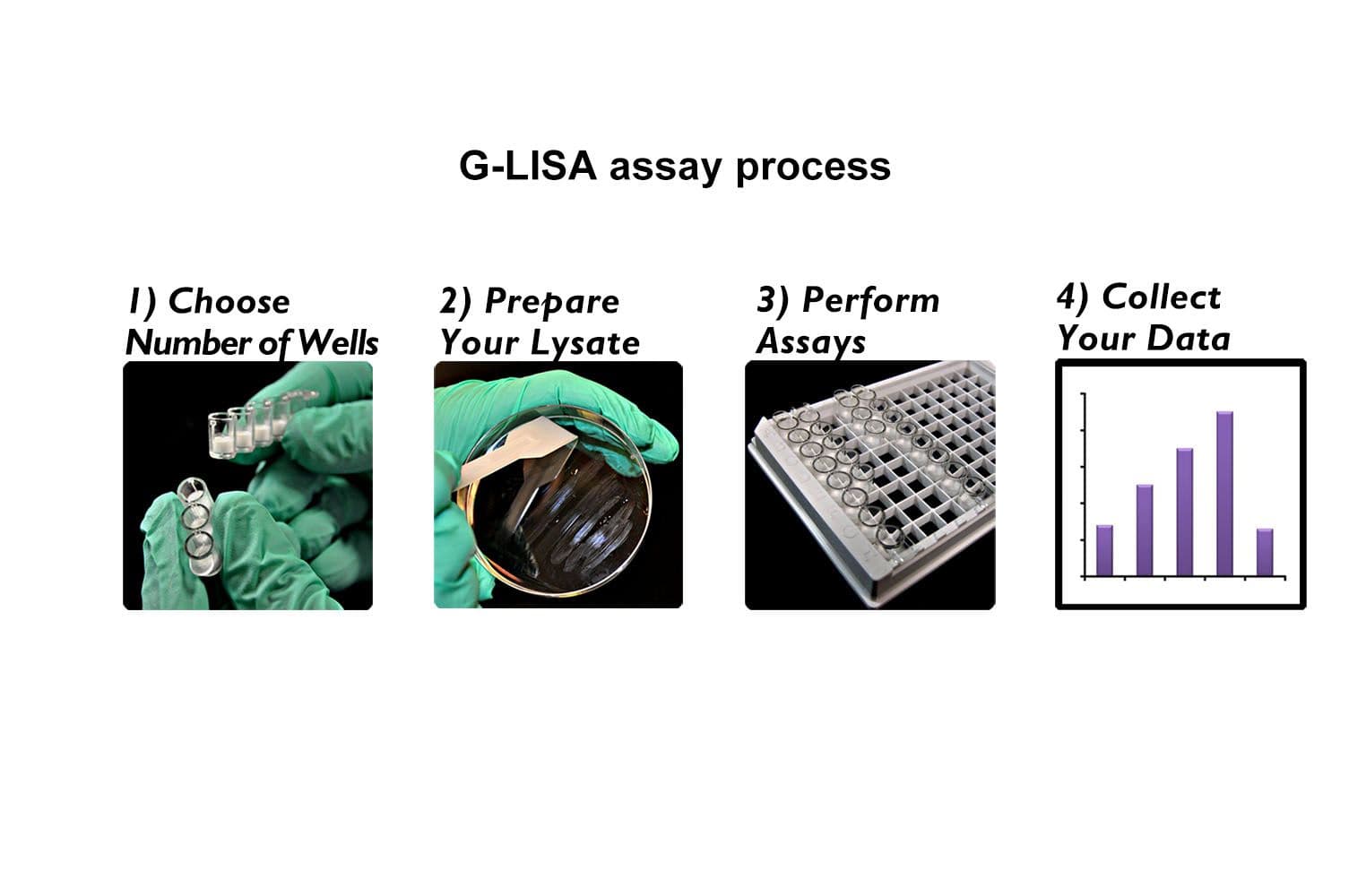
The Cdc42 G-LISA® assay isolates active Cdc42/Rac (GTP-bound form) using the Cdc42/Rac-binding domain (PBD) of an effector protein coupled to a 96-well strip plate. The bound Cdc42 is then detected and quantified using a colorimetric ELISA-based method and a Cdc42-specific antibody.
Key characteristics
Cat. #BK127