+3
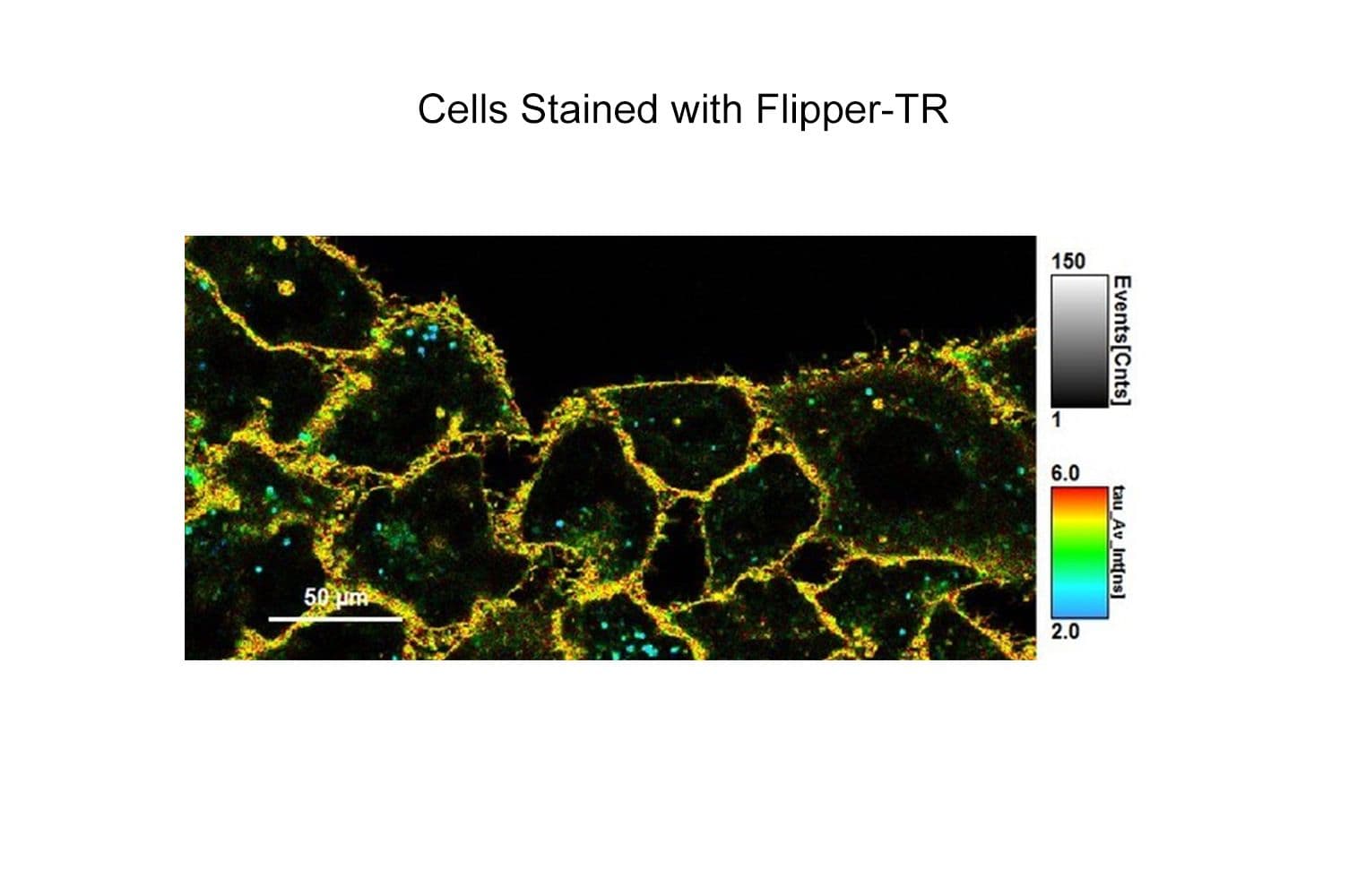
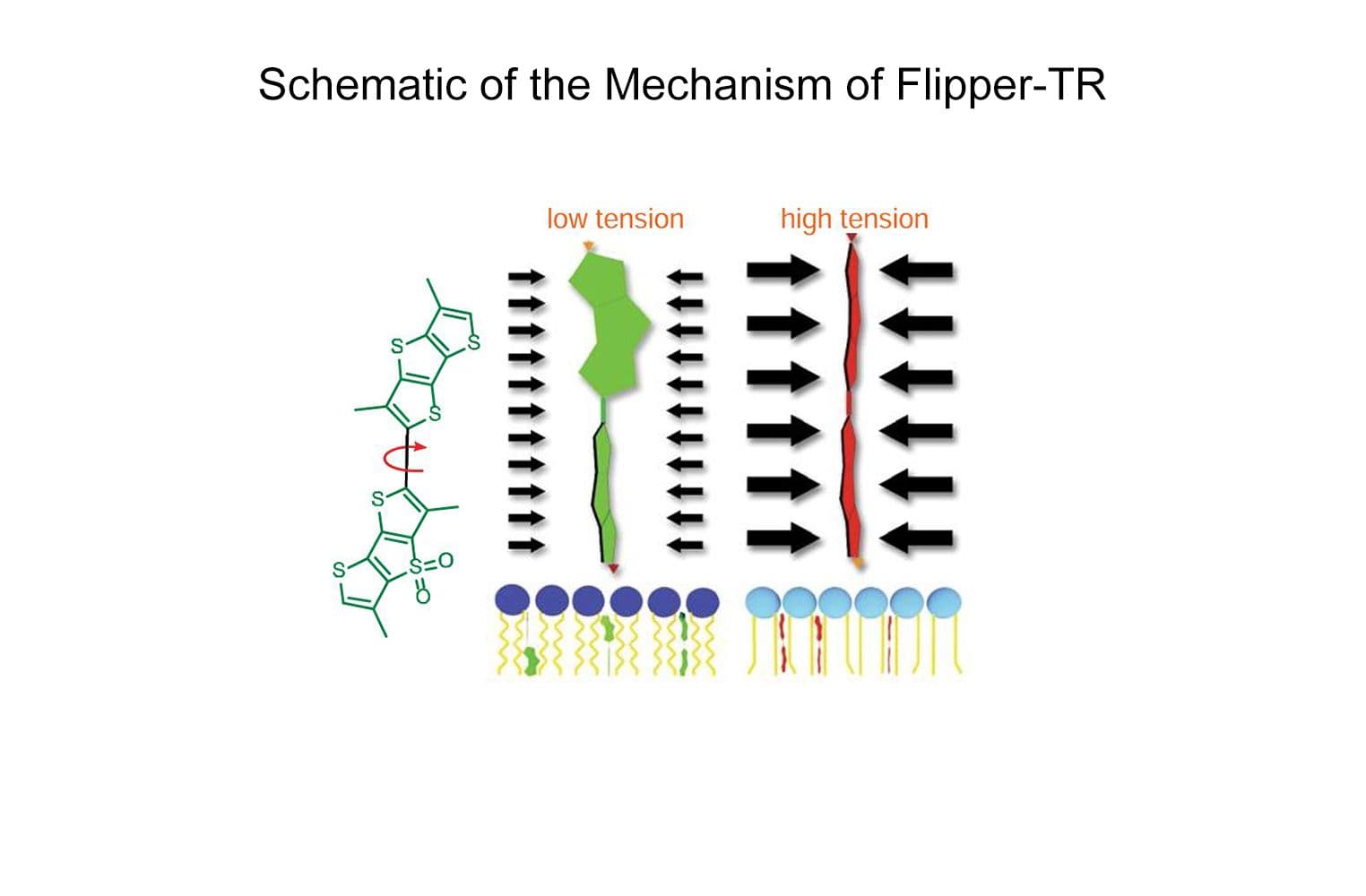
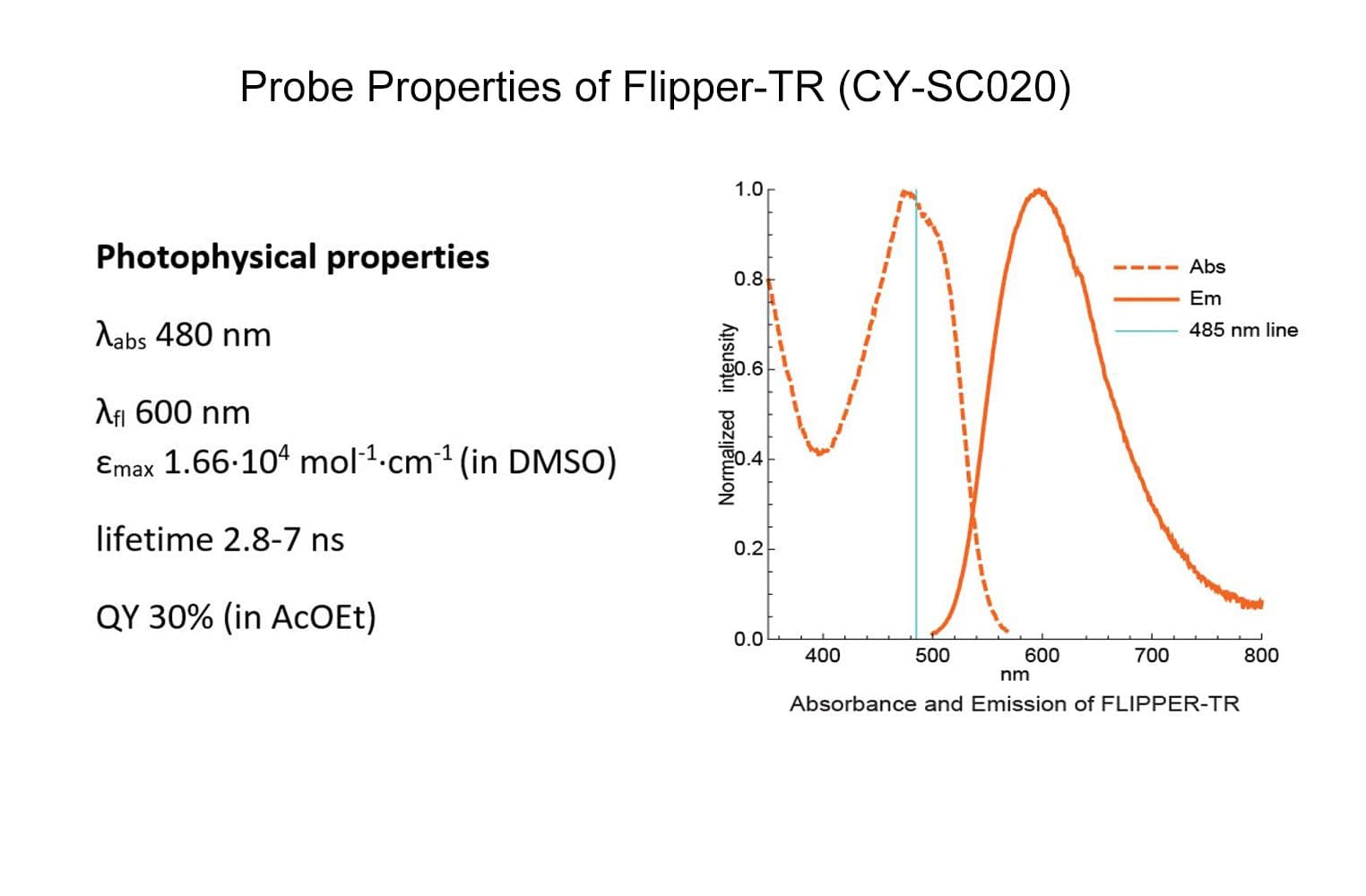
Flipper-TR® is a fluorogenic probe that specifically targets the plasma membrane of cells and reports membrane tension fluctuations through its fluorescence lifetime changes. It senses changes of the organization of lipid bilayer membranes through changes of the twist angle and polarization between the two twisted dithienothiophenes of the mechanophore. Flipper-TR spontaneously inserts into the plasma membrane of cells and is only fluorescent when inserted in a lipid membrane.
Key features
The biological activity of CY-SC020 is assessed by the ability of the probe to efficiently detect plasma membrane tension changes in live cell culture.
Cat. #CY-SC020