+3
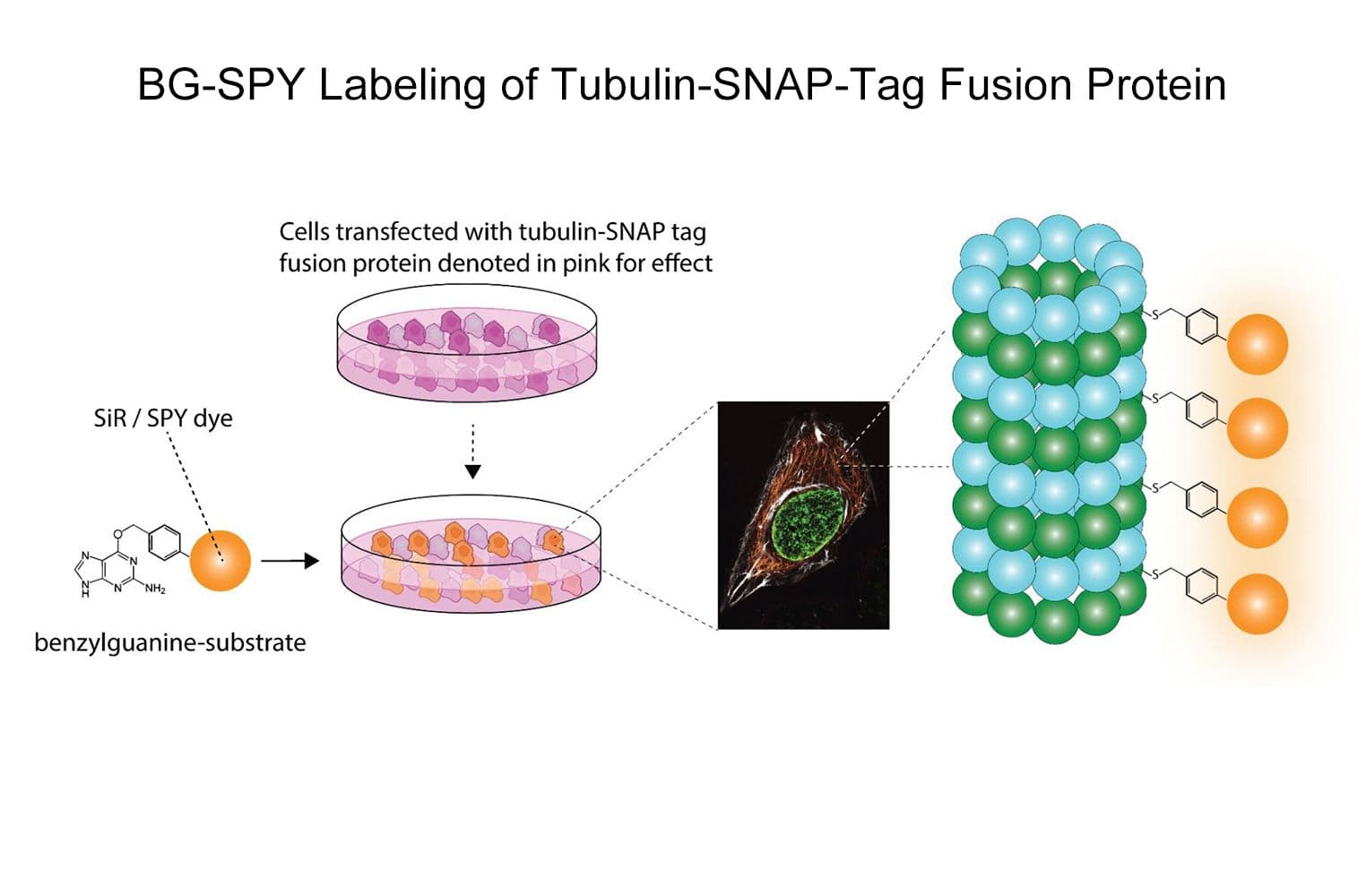
Substrate for SNAP-Tag®*: allows bright, fluorogenic, far-red visualization of any SNAP-tag® fusion protein of interest (POI) in live or fixed cells and tissues
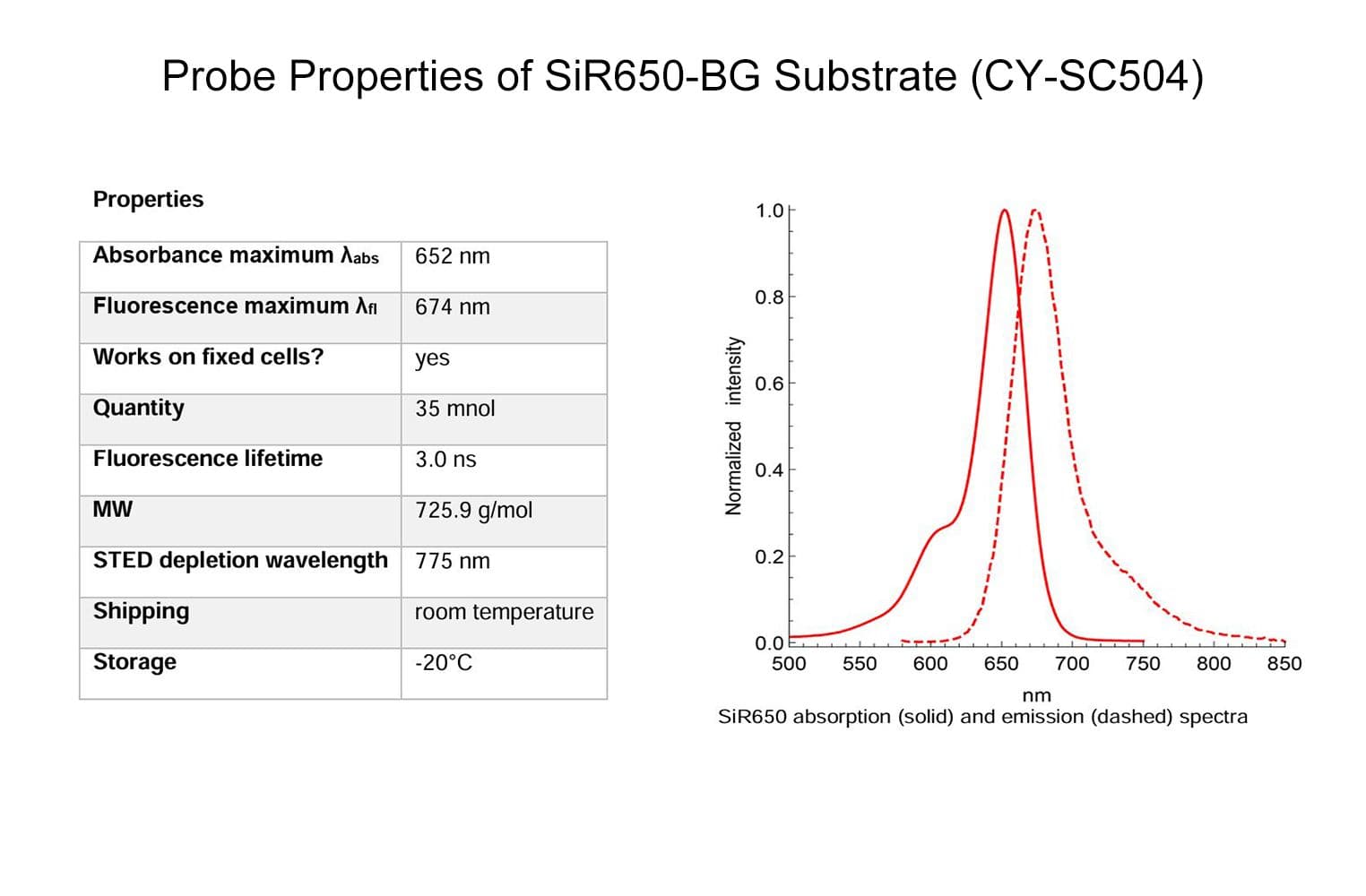
SiR650-BG is a benzylguanine (BG) derivative of the SiR fluorophore. BG is the substrate for SNAP-tag®, which forms a covalent bond upon reacting with it. This enables the permanent labeling of any POI that is expressed as a SNAP-tag® fusion, allowing fluorescent imaging of your POI in live or fixed cells.
Key features
SNAP-tag®is a registered trademark of New England Biolabs
Cat. #CY-SC504