Spirochrome Probes for Bioimaging
Live-cell imaging (SiR and SIR700) probes developed by Spirochrome are cell-permeable compounds which stain microtubules (SiR-Tubulin), F-actin (SiR-Actin), Lysosomes (SiR-Lysosome) and chromosomal DNA (SiR-DNA) in living cells.
Cytoskeleton is excited to introduce the next generation of Spirochrome’s live cell imaging probes - the SPY™ probes. SPY™ probes improve upon the SiR live cell imaging technology while also expanding the fluorophore labeling options for the study of F-actin, microtubules, and DNA in living cells.
Live cell F-actin imaging 2.0 with SPY650-FastAct™: This new probe is a unique fluorescent live cell actin probe that labels very dynamic actin filaments. Click Here to Learn More
Additional Spirochrome Probes and Tools:
Flipper-TR® is a live cell fluorescent membrane tension probe that simplifies the methodology for investigating changes in membrane tension when used in combination with standard fluorescence lifetime measurements.
Benzylguanine substrates enables investigators to label their SNAP-tagged-fused-protein of interest with these fluorescent dyes; thus, harnessing the exceptional qualities of the SPY™ and SiR probes to enhance their investigation and understanding of their target protein.
Click on the links below to learn more about these cutting-edge probes and tools for live cell imaging.
Cytoskeleton, Inc. is the exclusive provider of Spirochrome, Ltd. products in North America.
Spirochrome technology is based on the proprietary fluorophore silicon rhodamine (SiR). SiR is a bright and photostable rhodamine-like dye. Its key features are its cell permeability, fluorogenic character and compatibility with super-resolution microscopy The fluorescence excitation and emission of SiR are in the far-red, reducing phototoxicity in live-cell imaging experiments. SiR is compatible with most microscopes as it can be used with standard Cy5 settings. The combination of all these properties set SiR-based probes apart from other fluorescent probes. Read more in this Nature Chemistry paper about the properties of SiR.
Fluorogenic probes for live-cell imaging of the cytoskeleton
SiR-actin and SiR-tubulin were recently introduced in a landmark paper published in Nature Methods. The probes combine minimal cytotoxicity with excellent brightness for fluorescence imaging of actin and tubulin. Combined with super-resolution microscopy, SiR-actin and SiR-tubulin permit live-cell imaging of the cytoskeleton with unprecedented resolution.
SiR-actin and SiR-tubulin can be used without transfection and without washing steps. An experiment that highlights these features is the use of SiR-actin to stain the actin fibers of erythrocytes by simply adding SiR-actin to whole blood. Furthermore, their far-red excitation and emission spectra make them compatible with genetically encoded reporters.
References:
Spirochrome's live cell probes have been cited hundreds of times over the past several years. SiR-Actin citations are described here. More individual product citations are listed on each product page.
Featured Papers
“Fluorogenic probes for live-cell imaging of the cytoskeleton”; G. Lukinavičius, L.Reymond, E. D’Este, A. Masharina, F. Göttfert, H. Ta, A. Güther, M. Fournier, S. Rizzo, H. Waldmann, C. Blaukopf, C. Sommer, D. W. Gerlich, H.-D. Arndt, S. W. Hell & K. Johnsson; Nature Methods 11, 731–733, 2014.
“STED Nanoscopy Reveals the Ubiquity of Subcortical Cytoskeleton Periodicity in Living Neurons”; E. D’Este, D. Kamin, F. Göttfert, A. El-Hady, S. W. Hell; Cell Reports , Volume 10 , Issue 8 , 1246 – 1251, 2015.
“A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins”; G. Lukinavičius, K. Umezawa, N. Olivier, A. Honigmann, G. Yang, T. Plass, V. Mueller, L. Reymond, I. R. Corrêa Jr, Z. Luo, C. Schultz, E. A. Lemke, P. Heppenstall, C. Eggeling, S. Manley & K. Johnsson; Nature Chemistry 5, 132–139, 2013.
“Dynamic actin filaments control the mechanical behavior of the human red blood cell membrane”; D. S. Gokhin, R. B. Nowak, J. A. Khoory, A. de la Piedra, I. C. Ghiran and V. M. Fowler; Mol. Biol. Cell; February 25, 2015.
“A cleavable cytolysin-neuropeptide Y bioconjugate enables specific drug delivery and demonstrates intracellular mode of action”; V. M. Ahrens, K. B. Kostelnik, R. Rennert, D. Böhme, S. Kalkhof, D. Kosel, L. Weber, M. von Bergen and A. G. Beck-Sickinger; J. Control. Release; 209:170-178, 2015.
“Red Si–rhodamine drug conjugates enable imaging in GFP cells”; E. Kim, K. S. Yang, R. J. Giedt and R. Weissleder; Chem. Commun., 50, 4504-4507, 2014.
“A marginal band of microtubules transports and organizes mitochondria in retinal bipolar synaptic terminals”; M. Graffe, D. Zenisek, and J. Taraska; J. Gen Physiol. Vol. 146 No.1: 109-117, 2015.
Application Notes
“A Bright Dye for Live-Cell STED Microscopy”; S. Pitsch, I. Köster.
Q1. What is STED microscopy and how does it work?
A1. STED microscopy stands for Stimulated Emission Depletion microscopy. It is one type of super resolution microscopy which allows the capture of images with a higher resolution than conventional light microscopy which is constrained by diffraction of light. STED uses 2 laser pulses, one is the excitation pulse which excites the fluorophore, causing it to fluoresce. The second pulse, referred to as the STED pulse, de-excites the fluorophore via stimulated emission in an area surrounding a central focal spot that is not de-excited and thus continues to fluoresce. This is accomplished by focusing the STED pulse into a ring shape, a so-called donut, where the center focal spot is devoid of the STED laser pulse, conferring high resolution to the fluorescent area (Fig. 1; see Ref. 1 for more details on STED microscopy).
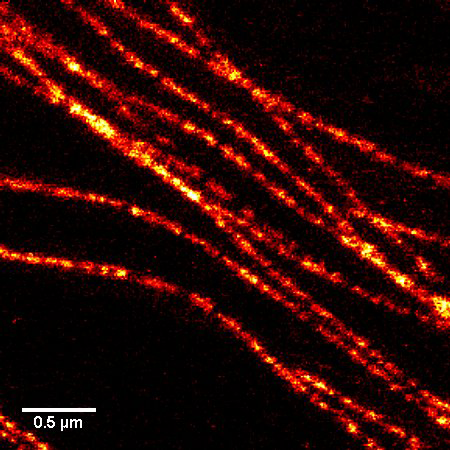
Figure 1. STED microscopic image of microtubules labeled with SiR-tubulin in human primary dermal fibroblasts.
Q2. Why is the SiR actin (or tubulin) probe good for STED microscopy?
A2. STED microscopy offers the ability to study cellular details on a nanometermolar scale in vivo. To take advantage of this super resolution microscopy, one must be able to select with high specificity the area to be examined using fluorescent probes. In addition, the fluorescent probes must be bright, photostable, exhibit no or little phototoxicity, be excited and emit in the far red spectrum. In addition, if the probe is to be used for live cell imaging (thus avoiding fixation artifacts that occur when cells are fixed), high cell permeability is necessary. The SiR actin and tubulin probes fulfill all of these requirements. In short, the combination of STED and SiR probes allows for unparalleled fluorescent visualization of subcellular actin and tubulin/microtubule structures and their physical characterization in living cells, (see Fig. 2 and Ref. 2).
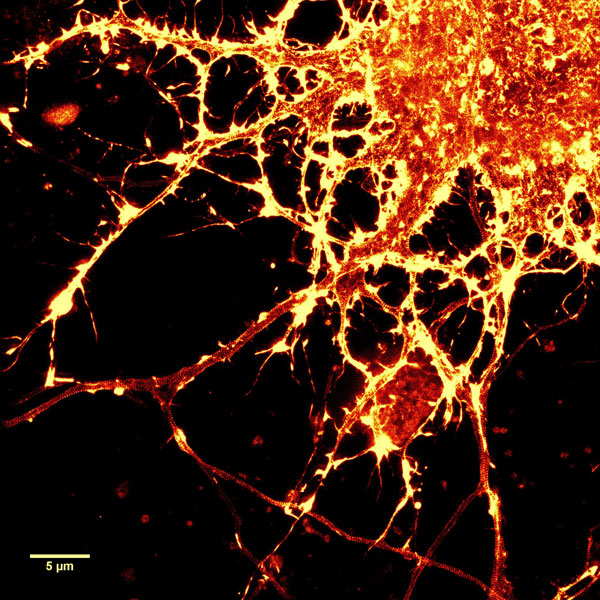
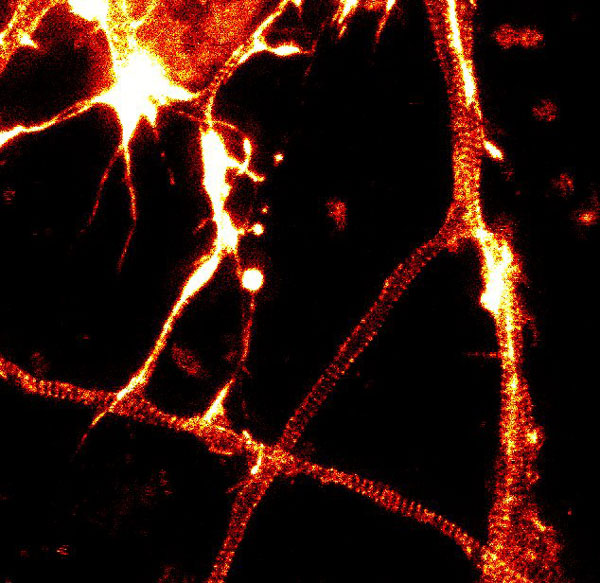
Figure 2. STED images of cultured rat hippocampal neurons stained with SiR-actin. Bottom image is a close-up view of part of the top image to clearly visualize actin rings (stripes) with 180 nm periodicity. Courtesy Of Elisa D'Este, MPI Biophysical Chemistry, Göttingen.
Q3. What are the filter sets for these probes?
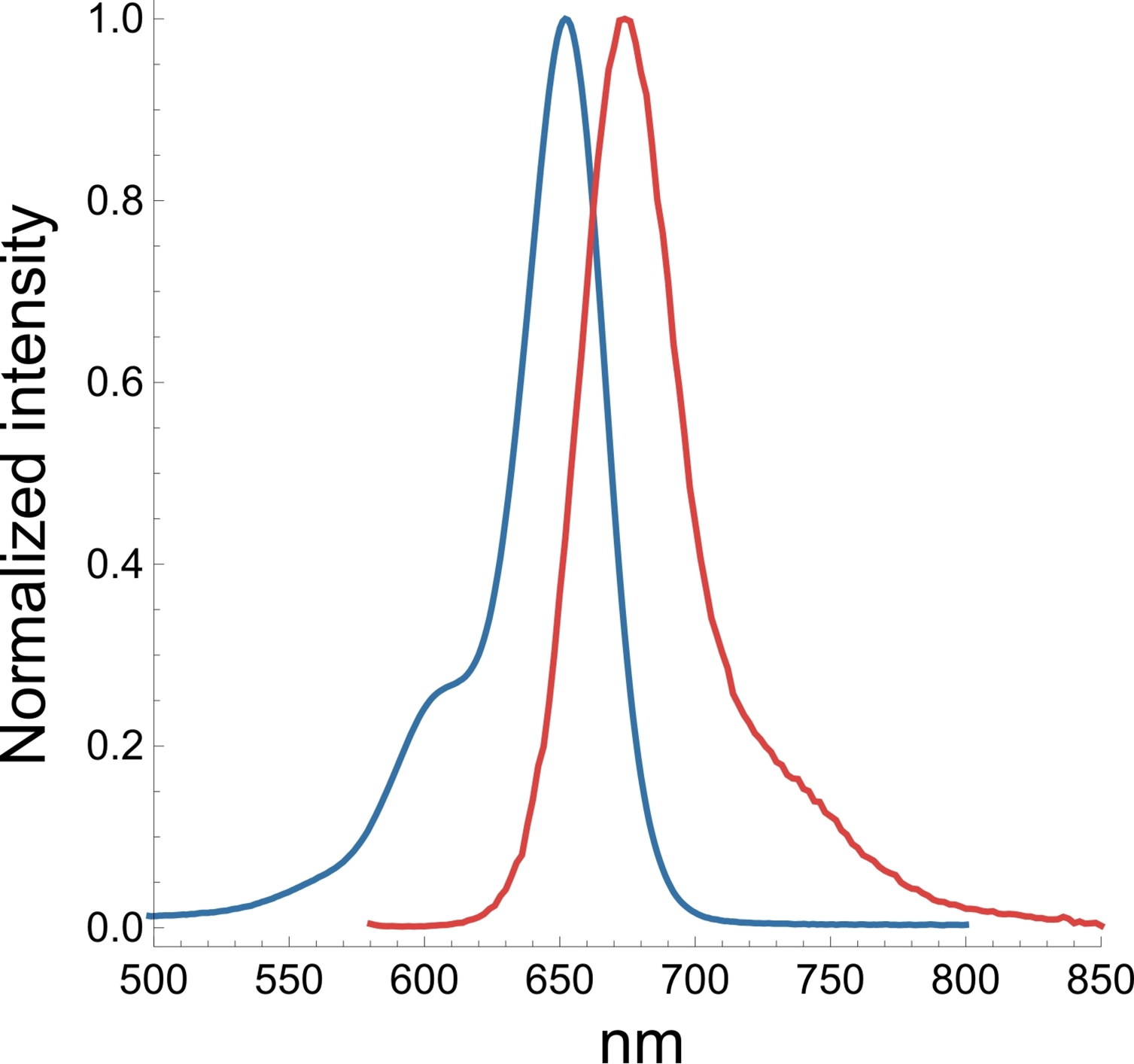
A3. The SiR actin and tubulin probes are visualized with standard Cy5 filters. Optimal excitation is 650 nm and emission is 670 nm. We recommend filters with an excitation of 630 + 20 nm and an emission of 680 + 20 nm (Fig. 3).
Q4. Why do the SiR probes have a low background compared to other fluorophores?
A4. SiR probes are excited by and emit light in the near infrared/far red spectral range, thus avoiding the use of shorter wavelengths such as blue and green light that typically autofluoresce, causing higher background signals. SiR-coupled probes possess two physical states: 1. a non-fluorescent, closed off-state (spirolactone) and 2. an open, highly fluorescent on-state (zwitterion). The binding of the probe to its ligand target favors the highly fluorescent open state while the free unbound probe exists in the closed, non-fluorescent state (Fig. 4). The fluorescence amplification is 100-fold from the unbound to bound state. This results in a highly sensitive biosensor in which the majority of fluorescence occurs only in the bound state (see Refs. 3 and 4).
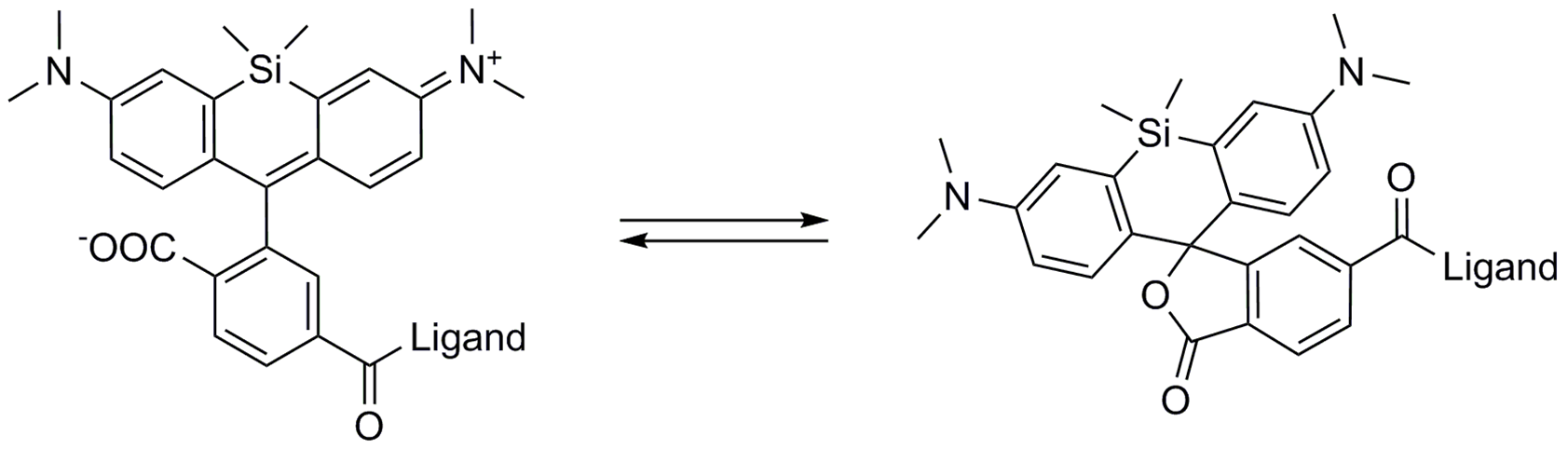
Figure 4. SiR derivatives exist in equilibrium between the fluorescent zwitterionic (open) form (left structure) and the non-fluorescent spiro (closed) form (right structure).
Q5: Are the SiR probes stable at room temperature?
A5: Yes, the probes are stable at room temperature for a few days. However, it strongly depends on the probe and the solvent. Thus, it is recommended to store all of the probes or solutions at –20°C.
Q6: Are SiR-actin and SiR-tubulin toxic to cells?
A6: Yes, above a certain threshold both probes show some effect on cell proliferation and altered actin or microtubule dynamics. However, the probes are orders of magnitude less toxic than their parent drug. In HeLa cells, neither actin nor microtubule dynamics were altered at concentrations below 100 nM. At this concentration, SiR probes efficiently label microtubules and F-actin, allowing for the capture of high signal to noise images.
Q7: Do the probes work on fixed cells?
A7: SiR-actin probes can be used with PFA-fixed cells. SiR-actin labels F-actin in PFA-fixed cells as efficiently as phalloidin derivatives. SiR-tubulin labels microtubules only in ethyleneglycol-bis-succinimidyl-succinate (EGS)-fixed cells. However, a selective labeling of centrosomal microtubules of PFA-fixed cells was observed. SiR-actin and SiR-tubulin are not suitable for methanol-fixed cells.
Q8: Is it possible to image SiR-probes by STORM?
A8: No—under the very high light intensities typically used in STORM imaging, a phototoxic effect is observed on live cells.
Q9: Which organisms and tissues are stained by SiR-probes?
A9: This list describes only cell lines, tissues, or organisms that have been reported to work. Omission of a cell line, tissue, or organism does not mean that the SiR-probes will not work with the specific cells, tissues, or organisms.
Homo sapiens: U2OS, fibroblasts, HeLa, HUVEC, MCF-10A, HCT-116, A549, erythrocytes
Mus musculus: C2C12, IA32, skeletal muscle, primary cardiomyocyte, primary oocyte
Rattus norvegicus: primary hippocampal neurons, primary cortical neurons, NRK
Cercopithecus aethiops: COS-7
Mesocricetus auratus: BHK
Drosophila melanogaster: Notum epithelium, S2
Didelphis marsupialis: OK cells
Q10. Do SiR-probes work in 3D cell cultures?
A10: Yes, the probes are able to stain cells in a 3D growth environment.
Q11: What are the correction factors CF260 and CF280 for the SiR fluorophore?
A11: CF260 = 0.116 and CF280 = 0.147
References
1. Hell S.W. and Wichmann J. 1994. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780-782.
2. D’Este E. et al. 2015. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 10, 1246-1251.
3. Lukinavicius G. et al. 2013. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 5, 132-139.
4. Lukinavicius G. et al. 2014. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nature Methods. 11, 731-733.