+3
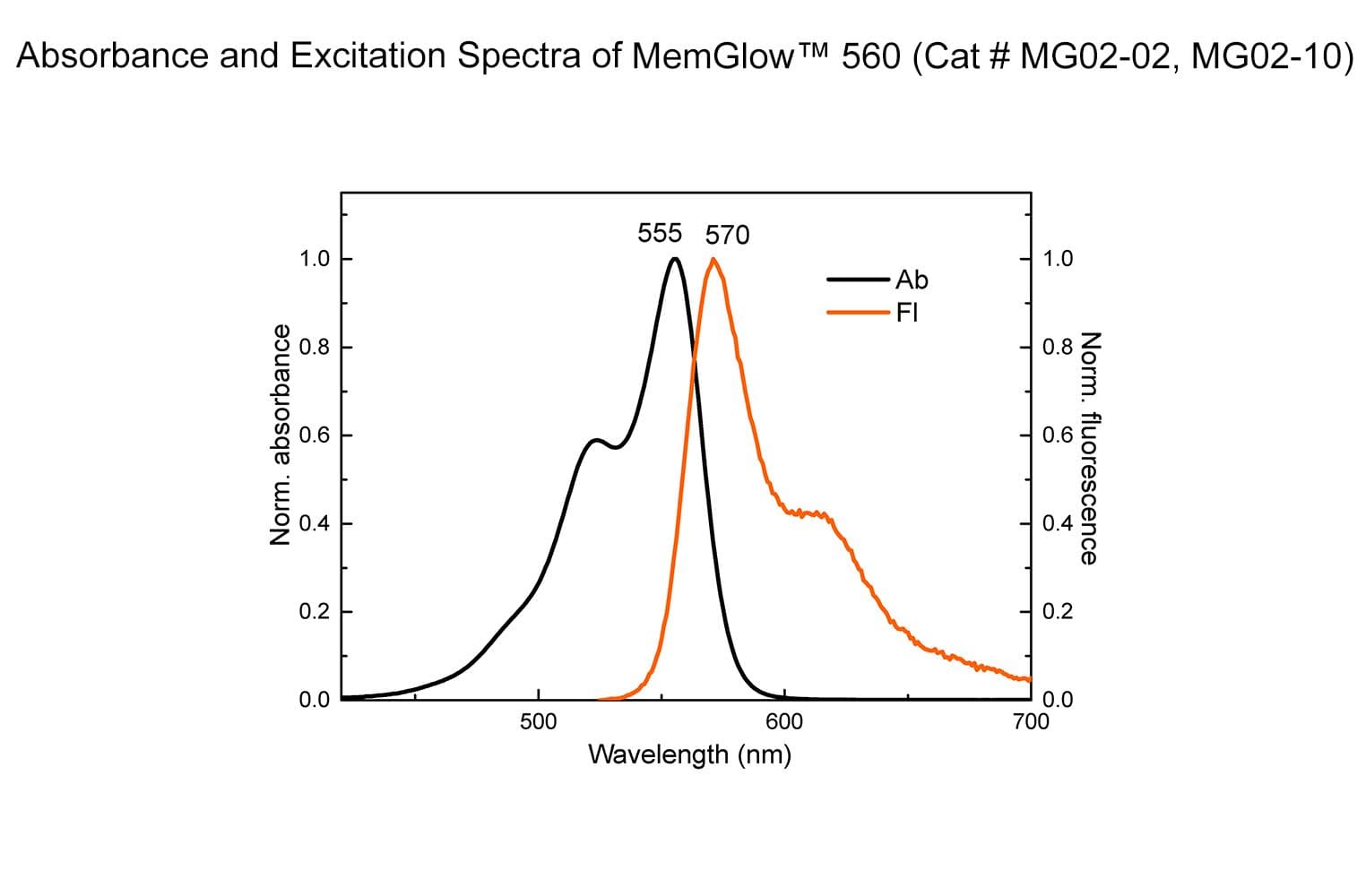
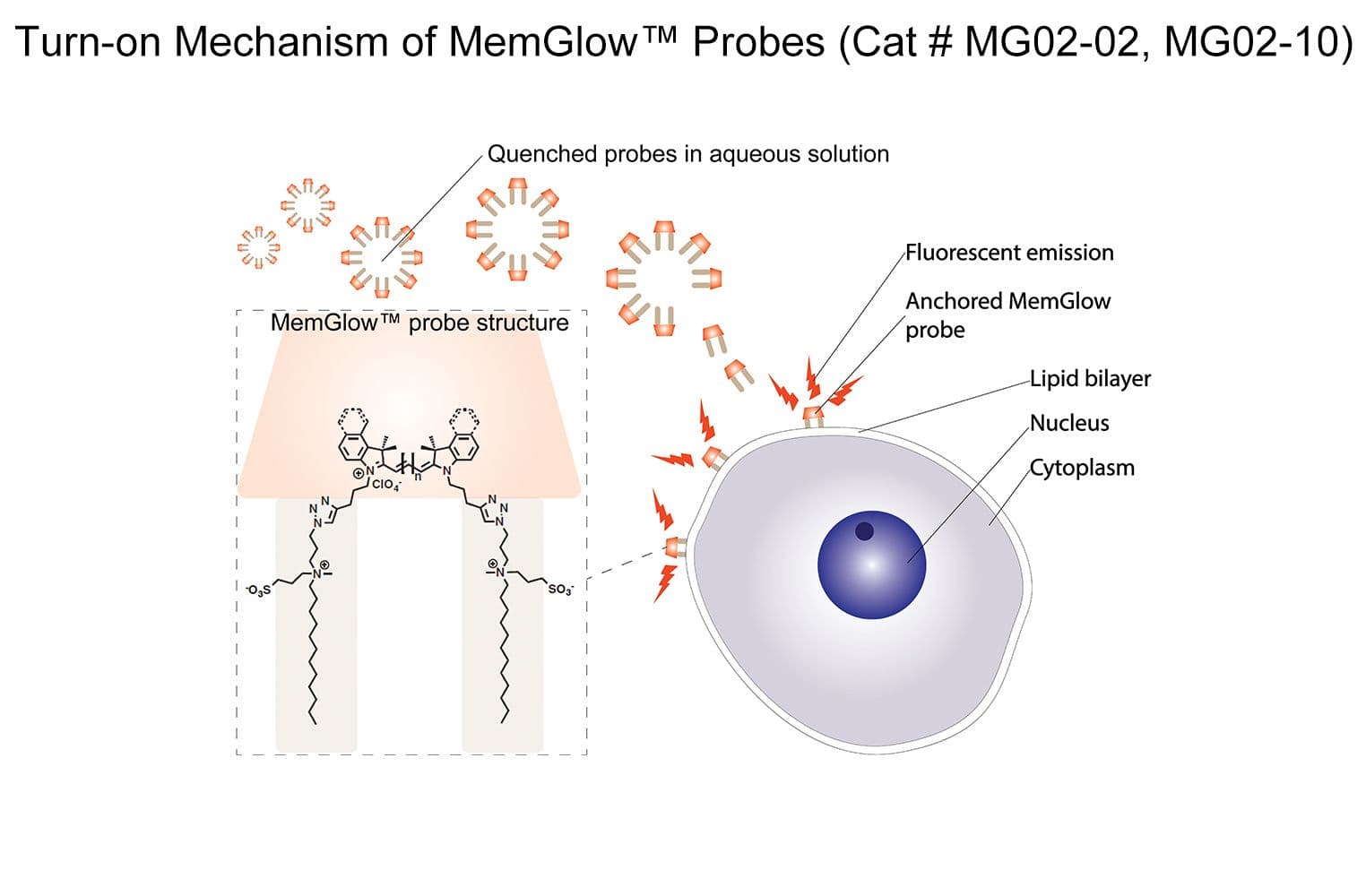
MemGlow™560 is a bright & nontoxic live cell membrane probe. It exhibits ideal microscopy characteristics, including high specificity, low background, and simple application
Key features
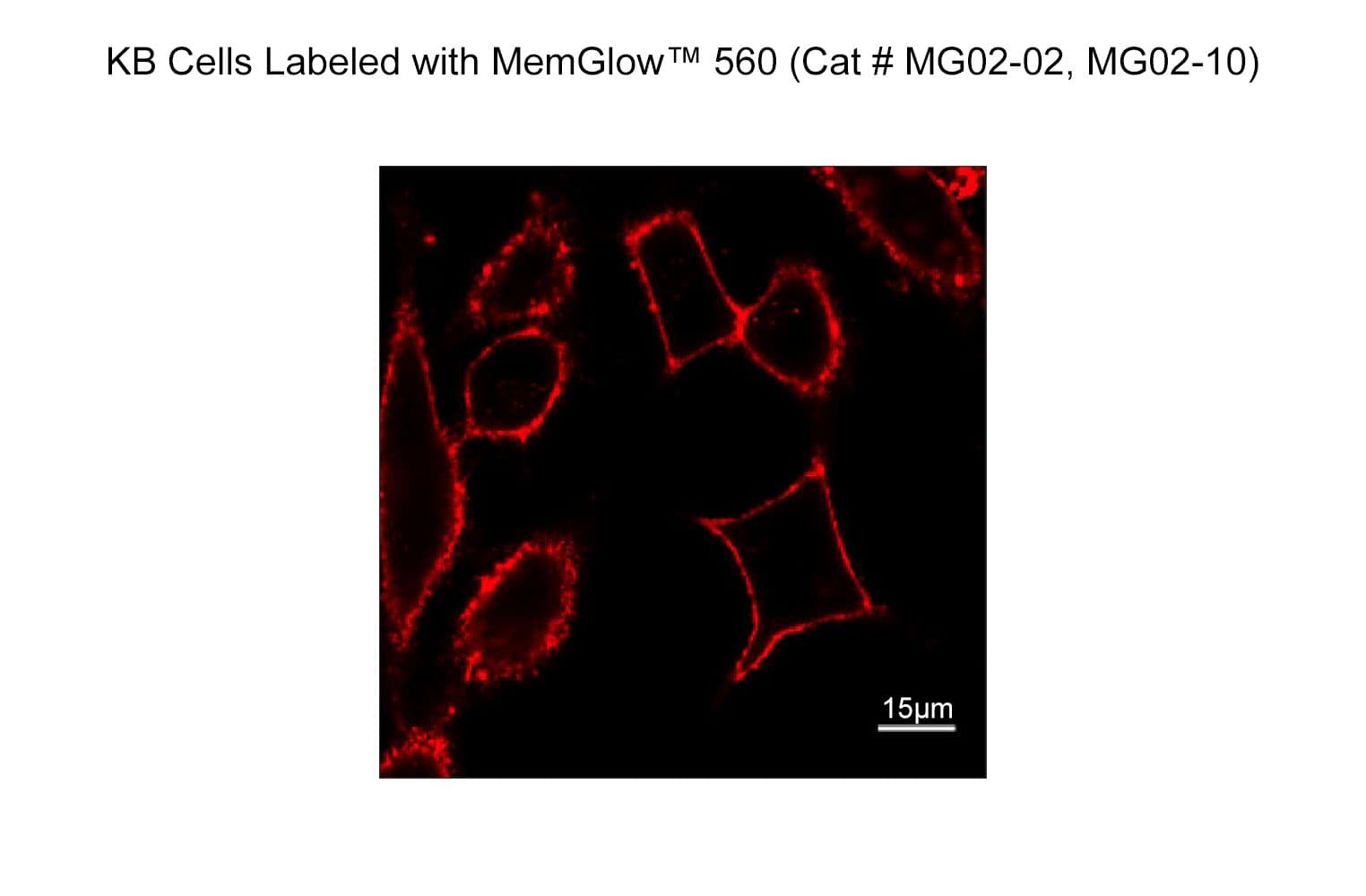
The biological activity of MG02 is assessed by the ability of the probe to efficiently label plasma membranes in live HEK293 and RAW 264.7 cell culture.
Cat. #MG02